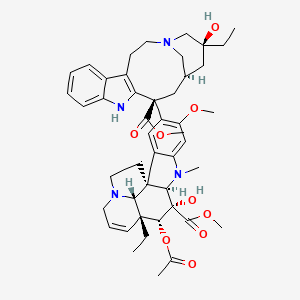
Vinblastine
"Antitumor alkaloid isolated from Vinca rosea. (Merck, 11th ed.)" (MeSH 2013)
Found this page useful?
 Web Resources: Vinblastine
Web Resources: Vinblastine Latest Research Publications
Latest Research PublicationsWeb Resources: Vinblastine (6 links)
Cancer Research UK
Macmillan Cancer Support
MedlinePlus
NHS Evidence
 Vinblastine - Substance Summary
Vinblastine - Substance Summary
PubChem
Irish Cancer Society
Latest Research Publications
This list of publications is regularly updated (Source: PubMed).
Ashraf SM, Sebastian J, Rathinasamy K
Zerumbone, a cyclic sesquiterpene, exerts antimitotic activity in HeLa cells through tubulin binding and exhibits synergistic activity with vinblastine and paclitaxel.
Cell Prolif. 2019; 52(2):e12558 [PubMed] Related Publications
Zerumbone, a cyclic sesquiterpene, exerts antimitotic activity in HeLa cells through tubulin binding and exhibits synergistic activity with vinblastine and paclitaxel.
Cell Prolif. 2019; 52(2):e12558 [PubMed] Related Publications
OBJECTIVES: The aim of this study was to elucidate the antimitotic mechanism of zerumbone and to investigate its effect on the HeLa cells in combination with other mitotic blockers.
MATERIALS AND METHODS: HeLa cells and fluorescence microscopy were used to analyse the effect of zerumbone on cancer cell lines. Cellular internalization of zerumbone was investigated using FITC-labelled zerumbone. The interaction of zerumbone with tubulin was characterized using fluorescence spectroscopy. The Chou and Talalay equation was used to calculate the combination index.
RESULTS: Zerumbone selectively inhibited the proliferation of HeLa cells with an IC
CONCLUSION: Our data suggest that disruption of microtubule assembly dynamics is one of the mechanisms of the anti-cancer activity of zerumbone and it can be used in combination therapy targeting cell division.
MATERIALS AND METHODS: HeLa cells and fluorescence microscopy were used to analyse the effect of zerumbone on cancer cell lines. Cellular internalization of zerumbone was investigated using FITC-labelled zerumbone. The interaction of zerumbone with tubulin was characterized using fluorescence spectroscopy. The Chou and Talalay equation was used to calculate the combination index.
RESULTS: Zerumbone selectively inhibited the proliferation of HeLa cells with an IC
CONCLUSION: Our data suggest that disruption of microtubule assembly dynamics is one of the mechanisms of the anti-cancer activity of zerumbone and it can be used in combination therapy targeting cell division.
Abdel-Malek R, Shohdy KS, Abbas N, et al.
Safety of Vinflunine in Patients with Advanced Urothelial Carcinoma Refractory to Platinum-based Chemotherapy: A Prospective Pilot Study.
Curr Drug Saf. 2019; 14(1):31-36 [PubMed] Related Publications
Safety of Vinflunine in Patients with Advanced Urothelial Carcinoma Refractory to Platinum-based Chemotherapy: A Prospective Pilot Study.
Curr Drug Saf. 2019; 14(1):31-36 [PubMed] Related Publications
BACKGROUND: Several single chemotherapeutic agents have been evaluated as the second-line treatment of advanced urothelial carcinoma. Despite encouraging efficacy outcomes, toxicity has often led to dose modifications or discontinuation. We aimed to assess the safety of vinflunine in a particular population of advanced transitional cell carcinoma of urothelium (TCCU), that were exposed to the previous toxicity of chemotherapy.
METHODS: This is an open-label, prospective, single-center pilot study to evaluate the response rate and safety profile of vinflunine in patients with advanced TCCU. It was planned to enroll 25 evaluable patients. Eligible patients are those with progressive disease after first-line platinum-based regimen for advanced or metastatic disease.
RESULTS: The study was prematurely closed due to two sudden deaths that were judged by the review board as treatment-related. Only ten patients were evaluated and received at least one cycle of vinflunine. All but one were male and seven underwent radical surgery. Eight had a distant metastasis (mainly lung and/or liver). Disease control rate was 40%, four patients had a partial response with median duration of response of 3.5 months. The median overall survival was 3.2 months (95% CI:1.67- 4.73). There were three serious adverse events namely two sudden deaths and one grade 4 thrombocytopenia. Nine grade 3/4 adverse events occurred. The most common all-grade adverse events were fatigue (50%), constipation (40%) and vomiting (40%). Moreover, grade 3 fatigue occurred in 30% of patients. Only one patient, who achieved PR for 5 months, was fit to receive further cytotoxic chemotherapy.
CONCLUSION: The activity of vinflunine in advanced urothelial carcinoma came at the expense of its safety. The use of vinflunine has to be limited to the selected group of patients. However, this is a single institute experience in a limited number of patients.
METHODS: This is an open-label, prospective, single-center pilot study to evaluate the response rate and safety profile of vinflunine in patients with advanced TCCU. It was planned to enroll 25 evaluable patients. Eligible patients are those with progressive disease after first-line platinum-based regimen for advanced or metastatic disease.
RESULTS: The study was prematurely closed due to two sudden deaths that were judged by the review board as treatment-related. Only ten patients were evaluated and received at least one cycle of vinflunine. All but one were male and seven underwent radical surgery. Eight had a distant metastasis (mainly lung and/or liver). Disease control rate was 40%, four patients had a partial response with median duration of response of 3.5 months. The median overall survival was 3.2 months (95% CI:1.67- 4.73). There were three serious adverse events namely two sudden deaths and one grade 4 thrombocytopenia. Nine grade 3/4 adverse events occurred. The most common all-grade adverse events were fatigue (50%), constipation (40%) and vomiting (40%). Moreover, grade 3 fatigue occurred in 30% of patients. Only one patient, who achieved PR for 5 months, was fit to receive further cytotoxic chemotherapy.
CONCLUSION: The activity of vinflunine in advanced urothelial carcinoma came at the expense of its safety. The use of vinflunine has to be limited to the selected group of patients. However, this is a single institute experience in a limited number of patients.
Bánóczi Z, Keglevich A, Szabó I, et al.
The effect of conjugation on antitumor activity of vindoline derivatives with octaarginine, a cell-penetrating peptide.
J Pept Sci. 2018; 24(10):e3118 [PubMed] Related Publications
The effect of conjugation on antitumor activity of vindoline derivatives with octaarginine, a cell-penetrating peptide.
J Pept Sci. 2018; 24(10):e3118 [PubMed] Related Publications
Some Vinca alkaloids (eg, vinblastine, vincristine) have been widely used as antitumor drugs for a long time. Unfortunately, vindoline, a main alkaloid component of Catharanthus roseus (L.) G. Don, itself, has no antitumor activity. In our novel research program, we have prepared and identified new vindoline derivatives with moderate cytostatic activity. Here, we describe the effect of conjugation of vindoline derivative with oligoarginine (tetra-, hexa-, or octapeptides) cell-penetrating peptides on the cytostatic activity in vitro and in vivo. Br-Vindoline-(l)-Trp-OH attached to the N-terminus of octaarginine was the most effective compound in vitro on HL-60 cell line. Analysis of the in vitro activity of two isomer conjugates (Br-vindoline-(l)-Trp-Arg
Jun HJ, Appleman VA, Wu HJ, et al.
A PDGFRα-driven mouse model of glioblastoma reveals a stathmin1-mediated mechanism of sensitivity to vinblastine.
Nat Commun. 2018; 9(1):3116 [PubMed] Free Access to Full Article Related Publications
A PDGFRα-driven mouse model of glioblastoma reveals a stathmin1-mediated mechanism of sensitivity to vinblastine.
Nat Commun. 2018; 9(1):3116 [PubMed] Free Access to Full Article Related Publications
Glioblastoma multiforme (GBM) is an aggressive primary brain cancer that includes focal amplification of PDGFRα and for which there are no effective therapies. Herein, we report the development of a genetically engineered mouse model of GBM based on autocrine, chronic stimulation of overexpressed PDGFRα, and the analysis of GBM signaling pathways using proteomics. We discover the tubulin-binding protein Stathmin1 (STMN1) as a PDGFRα phospho-regulated target, and that this mis-regulation confers sensitivity to vinblastine (VB) cytotoxicity. Treatment of PDGFRα-positive mouse and a patient-derived xenograft (PDX) GBMs with VB in mice prolongs survival and is dependent on STMN1. Our work reveals a previously unconsidered link between PDGFRα activity and STMN1, and highlight an STMN1-dependent cytotoxic effect of VB in GBM.
Banna GL, Camerini A, Bronte G, et al.
Oral Metronomic Vinorelbine in Advanced Non-small Cell Lung Cancer Patients Unfit for Chemotherapy.
Anticancer Res. 2018; 38(6):3689-3697 [PubMed] Related Publications
Oral Metronomic Vinorelbine in Advanced Non-small Cell Lung Cancer Patients Unfit for Chemotherapy.
Anticancer Res. 2018; 38(6):3689-3697 [PubMed] Related Publications
AIM: To explore the feasibility and activity of oral metronomic vinorelbine patients with advanced NSCLC not eligible to standard chemotherapy because of old age (≥70 years), and/or poor Eastern Cooperative Oncology Group performance status (≥2), and/or extensive brain or bone disease, and/or active comorbidities (≥2) requiring for pharmacological treatment.
PATIENTS AND METHODS: In a prospective phase II not randomized study, patients with stage IV NSCLC unfit to chemotherapy were treated with oral metronomic vinorelbine at 30 mg fixed dose three times a week until disease progression.
RESULTS: Fifty patients were treated, 19 (38%) in the first-line setting. Five patients (11%) experienced a grade 3 toxicity; no grade 4 toxicity occurred. Overall disease control rate was 32%, 44% and 26% in first and subsequent lines, respectively (p=0.39). Median OS and PFS were 7.3 months (95% confidence interval [CI]=4.7-10.0) and 2.7 months (95%CI=2.0-3.4), respectively.
CONCLUSION: These data support the activity and safety of metronomic vinorelbine in a relevant proportion of patients usually excluded from any specific treatment.
PATIENTS AND METHODS: In a prospective phase II not randomized study, patients with stage IV NSCLC unfit to chemotherapy were treated with oral metronomic vinorelbine at 30 mg fixed dose three times a week until disease progression.
RESULTS: Fifty patients were treated, 19 (38%) in the first-line setting. Five patients (11%) experienced a grade 3 toxicity; no grade 4 toxicity occurred. Overall disease control rate was 32%, 44% and 26% in first and subsequent lines, respectively (p=0.39). Median OS and PFS were 7.3 months (95% confidence interval [CI]=4.7-10.0) and 2.7 months (95%CI=2.0-3.4), respectively.
CONCLUSION: These data support the activity and safety of metronomic vinorelbine in a relevant proportion of patients usually excluded from any specific treatment.
Luchsinger C, Aguilar M, Burgos PV, et al.
Functional disruption of the Golgi apparatus protein ARF1 sensitizes MDA-MB-231 breast cancer cells to the antitumor drugs Actinomycin D and Vinblastine through ERK and AKT signaling.
PLoS One. 2018; 13(4):e0195401 [PubMed] Free Access to Full Article Related Publications
Functional disruption of the Golgi apparatus protein ARF1 sensitizes MDA-MB-231 breast cancer cells to the antitumor drugs Actinomycin D and Vinblastine through ERK and AKT signaling.
PLoS One. 2018; 13(4):e0195401 [PubMed] Free Access to Full Article Related Publications
Increasing evidence indicates that the Golgi apparatus plays active roles in cancer, but a comprehensive understanding of its functions in the oncogenic transformation has not yet emerged. At the same time, the Golgi is becoming well recognized as a hub that integrates its functions of protein and lipid biosynthesis to signal transduction for cell proliferation and migration in cancer cells. Nevertheless, the active function of the Golgi apparatus in cancer cells has not been fully evaluated as a target for combined treatment. Here, we analyzed the effect of perturbing the Golgi apparatus on the sensitivity of the MDA-MB-231 breast cancer cell line to the drugs Actinomycin D and Vinblastine. We disrupted the function of ARF1, a protein necessary for the homeostasis of the Golgi apparatus. We found that the expression of the ARF1-Q71L mutant increased the sensitivity of MDA-MB-231 cells to both Actinomycin D and Vinblastine, resulting in decreased cell proliferation and cell migration, as well as in increased apoptosis. Likewise, the combined treatment of cells with Actinomycin D or Vinblastine and Brefeldin A or Golgicide A, two disrupting agents of the ARF1 function, resulted in similar effects on cell proliferation, cell migration and apoptosis. Interestingly, each combined treatment had distinct effects on ERK1/2 and AKT signaling, as indicated by the decreased levels of either phospho-ERK1/2 or phospho-AKT. Our results suggest that disruption of Golgi function could be used as a strategy for the sensitization of cancer cells to chemotherapy.
Amiri B, Ahmadvand H, Farhadi A, et al.
Delivery of vinblastine-containing niosomes results in potent in vitro/in vivo cytotoxicity on tumor cells.
Drug Dev Ind Pharm. 2018; 44(8):1371-1376 [PubMed] Related Publications
Delivery of vinblastine-containing niosomes results in potent in vitro/in vivo cytotoxicity on tumor cells.
Drug Dev Ind Pharm. 2018; 44(8):1371-1376 [PubMed] Related Publications
Vinblastine (VB), as a chemotherapeutic agent, is widely used in treatment of different types of cancer. However, its clinical application is limited due to its low water solubility, side effects, and multidrug resistance. The aim of this study was to increase the therapeutic efficacy of VB using drug delivery systems. For this purpose, a PEGylated niosomal formulation of vinblastine (Pn-VB) was prepared by thin film hydration method and physicochemically characterized. Drug release pattern was performed by dialysis diffusion method. The cytotoxicity of Pn-VB was investigated against murine lung cancer TC-1 cells using MTT assay and its tumor inhibitory effect was evaluated in lung tumor-bearing C57BL/6 mice. Mean particle size, zeta potential, entrapment, and loading efficiency of niosomes were obtained to be about 234.3 ± 11.4 nm, -34.6 ± 4.2 mV, 99.92 ± 1.6%, and 2.673 ± 0.30%, respectively. While, the mean particle size and zeta potential for non-PEGylated niosomes were obtained about 212.4 nm and -31.4 mV, respectively. The in vitro release pattern of drug from niosomes showed a sustained release behavior. Pn-VB indicated a significant increase in toxicity against TC-l cells as compared to free VB. In animal model, Pn-VB exhibited stronger tumor inhibitory effect and longer life time in comparison to free VB. In conclusion, Pn-VB showed appropriate stability, high-entrapment efficacy, lower releasing rate, and stronger cytotoxic activity against lung cancer TC-1 cells as compared to free drug. Thus, the Pn-VB could be a promising formulation for delivery of vinblastine to tumor cells with enhanced drug bioavailability and therapeutic efficacy.
Ruf S, Hebart H, Hjalgrim LL, et al.
CNS progression during vinblastine or targeted therapies for high-risk relapsed ALK-positive anaplastic large cell lymphoma: A case series.
Pediatr Blood Cancer. 2018; 65(6):e27003 [PubMed] Related Publications
CNS progression during vinblastine or targeted therapies for high-risk relapsed ALK-positive anaplastic large cell lymphoma: A case series.
Pediatr Blood Cancer. 2018; 65(6):e27003 [PubMed] Related Publications
Vinblastine and targeted therapies induce remissions in patients with relapsed or progressive anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL). Central nervous system (CNS) prophylaxis often is not included during re-induction in CNS-negative relapse patients. We report on five patients with progressive or early relapsed ALK-positive ALCL who developed CNS progression during re-induction with vinblastine, crizotinib, or brentuximab vedotin given for bridging to allogeneic blood stem cell transplantation. These observations suggest that CNS prophylaxis should be considered in ALCL patients suffering progression during initial therapy who receive re-induction using agents with limited CNS penetration.
Sonobe M, Hamaji M, Motoyama H, et al.
Adjuvant vinorelbine and cisplatin after complete resection of stage II and III non-small cell lung cancer: long-term follow-up of our study of Japanese patients.
Surg Today. 2018; 48(7):687-694 [PubMed] Related Publications
Adjuvant vinorelbine and cisplatin after complete resection of stage II and III non-small cell lung cancer: long-term follow-up of our study of Japanese patients.
Surg Today. 2018; 48(7):687-694 [PubMed] Related Publications
PURPOSE: We reported previously a phase II study of adjuvant chemotherapy consisting of four cycles of vinorelbine (25 mg/m
METHODS: Between December 2006 and January 2011, 60 patients were enrolled in this study. We analyzed relapse-free and overall survival, long-lasting adverse effects, the influence of treatment on recurrent tumors, and the development of a second primary cancer, in relation with the regimen.
RESULTS: After a median follow-up period of 95.8 months, the 5-year relapse-free and overall survival rates were 51.7 and 76.7%, respectively. Neuralgia developed in one patient and this was the only case of a long-lasting adverse effect. Recurrence developed in 31 patients, 29 of whom received intensive treatment. Although 16 s (or more) primary neoplasms developed among 13 patients, these were common carcinomas in Japan and did not include sarcoma or hematologic malignancies.
CONCLUSION: Adjuvant vinorelbine and cisplatin chemotherapy showed encouraging relapse-free and overall survival rates, and long-term safety in Japanese patients with resected NSCLC.
METHODS: Between December 2006 and January 2011, 60 patients were enrolled in this study. We analyzed relapse-free and overall survival, long-lasting adverse effects, the influence of treatment on recurrent tumors, and the development of a second primary cancer, in relation with the regimen.
RESULTS: After a median follow-up period of 95.8 months, the 5-year relapse-free and overall survival rates were 51.7 and 76.7%, respectively. Neuralgia developed in one patient and this was the only case of a long-lasting adverse effect. Recurrence developed in 31 patients, 29 of whom received intensive treatment. Although 16 s (or more) primary neoplasms developed among 13 patients, these were common carcinomas in Japan and did not include sarcoma or hematologic malignancies.
CONCLUSION: Adjuvant vinorelbine and cisplatin chemotherapy showed encouraging relapse-free and overall survival rates, and long-term safety in Japanese patients with resected NSCLC.
Wu D, Wang L, Yang Y, et al.
MAD2-p31
Biochem Biophys Res Commun. 2018; 498(1):157-163 [PubMed] Related Publications
MAD2-p31
Biochem Biophys Res Commun. 2018; 498(1):157-163 [PubMed] Related Publications
Mitotic arrest deficient-like-1 (MAD2, also known as MAD2L1) is thought to be an important spindle assembly checkpoint protein, which ensures accurate chromosome segregation and is closely associated with poor prognosis in many cancer. As a MAD2 binding protein, p31
Palumbo R, Licata L, Sottotetti F, et al.
Vinflunine in Advanced Transitional Cell Cancer of the Urothelial Tract: A Potential Option for Maintenance Therapy? A Case Series.
Oncol Res Treat. 2018; 41(1-2):8-13 [PubMed] Related Publications
Vinflunine in Advanced Transitional Cell Cancer of the Urothelial Tract: A Potential Option for Maintenance Therapy? A Case Series.
Oncol Res Treat. 2018; 41(1-2):8-13 [PubMed] Related Publications
INTRODUCTION: Vinflunine is a microtubule inhibitor approved in Europe as second-line treatment of advanced transitional cell cancer of the urothelium (TCCU). The inability to continue with a first-line platinum-based regimen beyond 6 cycles suggested investigating the use of vinflunine as switch maintenance therapy in patients with response or stable disease after first-line therapy.
METHODS: Patients with advanced TCCU and documented disease control after 3-6 cycles of first-line platinum-based chemotherapy received vinflunine maintenance therapy within 6 weeks of the last cycle. Our analysis aimed to examine the performance of vinflunine in terms of activity and safety in such a patient population.
RESULTS: 28 consecutive patients were studied. After a median follow-up of 25 months, vinflunine was associated with a median progression-free survival of 9 months (range 4 to > 16 months) and a disease control rate of 64%; median overall survival was not reached. Treatment was well tolerated, with no unexpected safety events. The most common adverse events of grade ≥ 3 were neutropenia (21%) and constipation (14%); no toxicity-related death occurred.
CONCLUSIONS: Our results suggest that vinflunine may be a suitable maintenance treatment option for TCCU patients who received a maximum of 6 cycles of platinum-based chemotherapy commonly used as first-line treatment.
METHODS: Patients with advanced TCCU and documented disease control after 3-6 cycles of first-line platinum-based chemotherapy received vinflunine maintenance therapy within 6 weeks of the last cycle. Our analysis aimed to examine the performance of vinflunine in terms of activity and safety in such a patient population.
RESULTS: 28 consecutive patients were studied. After a median follow-up of 25 months, vinflunine was associated with a median progression-free survival of 9 months (range 4 to > 16 months) and a disease control rate of 64%; median overall survival was not reached. Treatment was well tolerated, with no unexpected safety events. The most common adverse events of grade ≥ 3 were neutropenia (21%) and constipation (14%); no toxicity-related death occurred.
CONCLUSIONS: Our results suggest that vinflunine may be a suitable maintenance treatment option for TCCU patients who received a maximum of 6 cycles of platinum-based chemotherapy commonly used as first-line treatment.
Hisakane K, Yoh K, Nakamura N, et al.
Salvage chemoradiotherapy with cisplatin and vinorelbine for postoperative locoregional recurrence of non-small cell lung cancer.
Medicine (Baltimore). 2017; 96(47):e8635 [PubMed] Free Access to Full Article Related Publications
Salvage chemoradiotherapy with cisplatin and vinorelbine for postoperative locoregional recurrence of non-small cell lung cancer.
Medicine (Baltimore). 2017; 96(47):e8635 [PubMed] Free Access to Full Article Related Publications
Although a few investigators have demonstrated the effect of concurrent chemoradiotherapy (CRT) for postoperative recurrent non-small cell lung cancer (NSCLC), the outcome of this treatment remains unclear. The aim of this study was to elucidate the efficacy and tolerability of concurrent CRT with cisplatin (CDDP) and vinorelbine (VNR) in patients with postoperative locoregional recurrent NSCLC. A total of 40 patients who had received concurrent CRT with CDDP and VNR between January 1999 and December 2014 were retrospectively analyzed. Patients were treated with CDDP (80 mg/m on day 1) and VNR (20 mg/m on days 1 and 8) every 4 weeks. Radiotherapy was administered concurrently during cycle 1. The delivered x-ray radiation dose was 60 Gy in all 37 patients who received x-ray radiotherapy; 3 patients received proton beam radiation (66 Gy [RBE] in 1 patient and 60 Gy [RBE] in 2 patients). The objective response rate was 85% (95% confidence interval [CI], 70.9%-92.9%). The median progression-free survival was 20.3 months (95% CI, 12.9 months-not reached). The 2-year survival rate was 78.9% (95% CI, 63.0%-89.1%). The most common grade ≥3 toxicity was neutropenia (18%). No grade ≥3 radiation pneumonitis and no treatment-related deaths were observed.Our study revealed that concurrent CRT with CDDP and VNR was active and safe for patients with postoperative locoregional recurrent NSCLC. Salvage CRT for postoperative locoregional recurrent NSCLC might be a promising treatment for selected patients.
Chen TW, Yeh DC, Chao TY, et al.
A Phase I/II study of the combination of lapatinib and oral vinorelbine in HER2-positive metastatic breast cancer.
Jpn J Clin Oncol. 2018; 48(3):242-247 [PubMed] Related Publications
A Phase I/II study of the combination of lapatinib and oral vinorelbine in HER2-positive metastatic breast cancer.
Jpn J Clin Oncol. 2018; 48(3):242-247 [PubMed] Related Publications
Background: The combination of lapatinib and oral vinorelbine for HER2 positive metastatic breast cancer (MBC) is convenient but with uncertain toxicity profiles. A Phase I/II study was designed to understand the tolerability and efficacy of this combination treatment.
Method: Female MBC patients with HER2 positive were eligible. Lapatinib was given once daily and oral vinorelbine was given on Days 1 and 8 of a 21-day cycle. A 3 + 3 standard dose-escalation rule was applied in the Phase I study. The primary endpoint of the Phase II study was PFS. In the Phase II part, because no DLT was observed in the first 20 patients, vinorelbine dose-escalation was permitted if no significant toxicities after the first cycle was observed.
Result: From June 2009 to February 2013, 46 patients were enrolled in Phase I (n = 15) and II (n = 31) studies. Median age was 52.8 (range 34.3-84.0); 28 (60.9%) patients were ER positive. In the Phase I study, two patients had DLTs (neutropenia (n = 2), diarrhea (n = 1)). The MTD was determined at lapatinib 1000 mg plus oral vinorelbine 50 mg/m2. In the Phase II study, 11 patients safely had vinorelbine escalated to 60 mg/m2 on cycle 2. The median PFS was 5.6 months (95% CI 5.2-5.9); 6 (19.4%) patients had PR; the clinical benefit rate was 38.7%. Six patients had disease control over 2 years.
Conclusion: Lapatinib 1000 mg and oral vinorelbine 50 mg/m2 were tolerable with manageable toxicities. Escalation to vinorelbine 60 mg/m2 is feasible if no significant toxicities after the first cycle. Clinical efficacy was demonstrated with long-term responders observed.
Method: Female MBC patients with HER2 positive were eligible. Lapatinib was given once daily and oral vinorelbine was given on Days 1 and 8 of a 21-day cycle. A 3 + 3 standard dose-escalation rule was applied in the Phase I study. The primary endpoint of the Phase II study was PFS. In the Phase II part, because no DLT was observed in the first 20 patients, vinorelbine dose-escalation was permitted if no significant toxicities after the first cycle was observed.
Result: From June 2009 to February 2013, 46 patients were enrolled in Phase I (n = 15) and II (n = 31) studies. Median age was 52.8 (range 34.3-84.0); 28 (60.9%) patients were ER positive. In the Phase I study, two patients had DLTs (neutropenia (n = 2), diarrhea (n = 1)). The MTD was determined at lapatinib 1000 mg plus oral vinorelbine 50 mg/m2. In the Phase II study, 11 patients safely had vinorelbine escalated to 60 mg/m2 on cycle 2. The median PFS was 5.6 months (95% CI 5.2-5.9); 6 (19.4%) patients had PR; the clinical benefit rate was 38.7%. Six patients had disease control over 2 years.
Conclusion: Lapatinib 1000 mg and oral vinorelbine 50 mg/m2 were tolerable with manageable toxicities. Escalation to vinorelbine 60 mg/m2 is feasible if no significant toxicities after the first cycle. Clinical efficacy was demonstrated with long-term responders observed.
Zhong WZ, Wang Q, Mao WM, et al.
Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study.
Lancet Oncol. 2018; 19(1):139-148 [PubMed] Related Publications
Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study.
Lancet Oncol. 2018; 19(1):139-148 [PubMed] Related Publications
BACKGROUND: Cisplatin-based adjuvant chemotherapy is the standard of care for patients with resected stage II-IIIA non-small-cell lung cancer (NSCLC). RADIANT and SELECT trial data suggest patients with EGFR-mutant stage IB-IIIA resected NSCLC could benefit from adjuvant EGFR tyrosine kinase inhibitor treatment. We aimed to compare the efficacy of adjuvant gefitinib versus vinorelbine plus cisplatin in patients with completely resected EGFR-mutant stage II-IIIA (N1-N2) NSCLC.
METHODS: We did a randomised, open-label, phase 3 trial at 27 centres in China. We enrolled patients aged 18-75 years with completely resected (R0), stage II-IIIA (N1-N2), EGFR-mutant (exon 19 deletion or exon 21 Leu858Arg) NSCLC. Patients were stratified by N stage and EGFR mutation status and randomised (1:1) by Pocock and Simon minimisation with a random element to either gefitinib (250 mg once daily) for 24 months or intravenous vinorelbine (25 mg/m
FINDINGS: Between Sept 19, 2011, and April 24, 2014, 483 patients were screened and 222 patients were randomised, 111 to gefitinib and 111 to vinorelbine plus cisplatin. Median follow-up was 36·5 months (IQR 23·8-44·8). Median disease-free survival was significantly longer with gefitinib (28·7 months [95% CI 24·9-32·5]) than with vinorelbine plus cisplatin (18·0 months [13·6-22·3]; hazard ratio [HR] 0·60, 95% CI 0·42-0·87; p=0·0054). In the safety population, the most commonly reported grade 3 or worse adverse events in the gefitinib group (n=106) were raised alanine aminotransferase and asparate aminotransferase (two [2%] patients with each event vs none with vinorelbine plus cisplatin). In the vinorelbine plus cisplatin group (n=87), the most frequently reported grade 3 or worse adverse events were neutropenia (30 [34%] patients vs none with gefitinib), leucopenia (14 [16%] vs none), and vomiting (eight [9%] vs none). Serious adverse events were reported for seven (7%) patients who received gefitinib and 20 (23%) patients who received vinorelbine plus cisplatin. No interstitial lung disease was noted with gefitinib. No deaths were treatment related.
INTERPRETATION: Adjuvant gefitinib led to significantly longer disease-free survival compared with that for vinorelbine plus cisplatin in patients with completely resected stage II-IIIA (N1-N2) EGFR-mutant NSCLC. Based on the superior disease-free survival, reduced toxicity, and improved quality of life, adjuvant gefitinib could be a potential treatment option compared with adjuvant chemotherapy in these patients. However, the duration of benefit with gefitinib after 24 months might be limited and overall survival data are not yet mature.
FUNDING: Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine; National Health and Family Planning Commission of People's Republic of China; Guangzhou Science and Technology Bureau; AstraZeneca China.
METHODS: We did a randomised, open-label, phase 3 trial at 27 centres in China. We enrolled patients aged 18-75 years with completely resected (R0), stage II-IIIA (N1-N2), EGFR-mutant (exon 19 deletion or exon 21 Leu858Arg) NSCLC. Patients were stratified by N stage and EGFR mutation status and randomised (1:1) by Pocock and Simon minimisation with a random element to either gefitinib (250 mg once daily) for 24 months or intravenous vinorelbine (25 mg/m
FINDINGS: Between Sept 19, 2011, and April 24, 2014, 483 patients were screened and 222 patients were randomised, 111 to gefitinib and 111 to vinorelbine plus cisplatin. Median follow-up was 36·5 months (IQR 23·8-44·8). Median disease-free survival was significantly longer with gefitinib (28·7 months [95% CI 24·9-32·5]) than with vinorelbine plus cisplatin (18·0 months [13·6-22·3]; hazard ratio [HR] 0·60, 95% CI 0·42-0·87; p=0·0054). In the safety population, the most commonly reported grade 3 or worse adverse events in the gefitinib group (n=106) were raised alanine aminotransferase and asparate aminotransferase (two [2%] patients with each event vs none with vinorelbine plus cisplatin). In the vinorelbine plus cisplatin group (n=87), the most frequently reported grade 3 or worse adverse events were neutropenia (30 [34%] patients vs none with gefitinib), leucopenia (14 [16%] vs none), and vomiting (eight [9%] vs none). Serious adverse events were reported for seven (7%) patients who received gefitinib and 20 (23%) patients who received vinorelbine plus cisplatin. No interstitial lung disease was noted with gefitinib. No deaths were treatment related.
INTERPRETATION: Adjuvant gefitinib led to significantly longer disease-free survival compared with that for vinorelbine plus cisplatin in patients with completely resected stage II-IIIA (N1-N2) EGFR-mutant NSCLC. Based on the superior disease-free survival, reduced toxicity, and improved quality of life, adjuvant gefitinib could be a potential treatment option compared with adjuvant chemotherapy in these patients. However, the duration of benefit with gefitinib after 24 months might be limited and overall survival data are not yet mature.
FUNDING: Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine; National Health and Family Planning Commission of People's Republic of China; Guangzhou Science and Technology Bureau; AstraZeneca China.
Jaferian S, Soleymaninejad M, Daraee H
Verapamil (VER) Enhances the Cytotoxic Effects of Docetaxel and Vinblastine Combined Therapy Against Non-Small Cell Lung Cancer Cell Lines.
Drug Res (Stuttg). 2018; 68(3):146-152 [PubMed] Related Publications
Verapamil (VER) Enhances the Cytotoxic Effects of Docetaxel and Vinblastine Combined Therapy Against Non-Small Cell Lung Cancer Cell Lines.
Drug Res (Stuttg). 2018; 68(3):146-152 [PubMed] Related Publications
Lung cancer is one of the foremost tumor-associated cause of death in the world. Most of the patients with NSCLC possesses an advanced disease at diagnosis, and are thus probable subject for systemic therapy. This study aims to evaluate the cytotoxicity of vinblastine and docetaxel combined therapy for the treatment of NSCLC, as well as verapamil (VER) enhancement of the combined therapy. We conducted P-glycoprotein (P-gp) gene expression, protein expression with RT-PCR and western blot respectively, apoptotic response of the combined therapy with VER is also determined using DAPI staining (%). Result of DAPI staining confirmed combination therapy promotes cell apoptosis to greater extent as compared to each drug alone. Real-time RT-PCR analysis revealed that mdr-1 expression level increased 6 fold with docetaxel (40 nM) and 2 fold with vinblastine (30 nM) after 24 h (p<0.001). Consequently, combination therapy reduced drug-induced up-regulation of mdr-1 significantly (p<0.05). VER with the drug combination increased P-gp expression (p<0.05). These data provide evidence showing combined therapy is a better approach to improve the efficacy of chemotherapy and decreasing drug resistance.
Schinzari G, Rossi E, Cassano A, et al.
Cisplatin, dacarbazine and vinblastine as first line chemotherapy for liver metastatic uveal melanoma in the era of immunotherapy: a single institution phase II study.
Melanoma Res. 2017; 27(6):591-595 [PubMed] Related Publications
Cisplatin, dacarbazine and vinblastine as first line chemotherapy for liver metastatic uveal melanoma in the era of immunotherapy: a single institution phase II study.
Melanoma Res. 2017; 27(6):591-595 [PubMed] Related Publications
No standard therapy is established for metastatic uveal melanoma. Liver involvement in uveal melanoma may lead to organ impairment, which represents a common cause of death. Tumor shrinkage might improve survival by delaying hepatic failure. Since the combination of cisplatin, vinblastine, dacarbazine allowed a high response rate in metastatic cutaneous melanoma, we explored efficacy and safety of this regimen in unresectable liver metastases of uveal melanoma. In the present phase II study we administered intravenously cisplatin (80 mg/mq, day 1), dacarbazine (250 mg/mq/day, days 1-3), vinblastine (2 mg maximum, day 1) every 21 days as first line treatment for patients with unresectable metastases of uveal melanoma and BRAF wild type. Primary endpoint was objective response rate; overall survival (OS), progression-free survival and toxicity were secondary endpoints. Partial responses were observed in five (20%) patients, stable disease in 12 (48%) patients; disease control rate was 68%. Median OS of all the patients was 13 months, median progression free survival was 5.5 months. OS of responding patients was 21 months; OS of patients with disease control was 18 months, significantly longer than survival of progressing patients (7 months, P=0.0003). Five (20%) patients experienced grade 3-4 toxicity. Combination of cisplatin, vinblastine and dacarbazine was feasible and demonstrate both an interesting objective response rate and a survival benefit for patients achieving a disease control. This regimen could be considered for patients with good performance status and unresectable liver limited disease.
Heers H, DE Geeter P, Goebell PJ, et al.
Vinflunine in the Treatment of Upper Tract Urothelial Carcinoma - Subgroup Analysis of an Observational Study.
Anticancer Res. 2017; 37(11):6437-6442 [PubMed] Related Publications
Vinflunine in the Treatment of Upper Tract Urothelial Carcinoma - Subgroup Analysis of an Observational Study.
Anticancer Res. 2017; 37(11):6437-6442 [PubMed] Related Publications
BACKGROUND/AIM: Despite an expected prognostic disadvantage for upper tract versus lower tract metastatic urothelial carcinomas (UTUC/LTUC), only few studies have been conducted to elucidate potential differences in chemotherapy treatment.
PATIENTS AND METHODS: A post-hoc subgroup analysis of a non-interventional study investigating vinflunine after failure of a platinum-based chemotherapy in metastatic/locally advanced UC patients was performed.
RESULTS: A total of 18 and 59 out of 77 patients had UTUC and LTUC, respectively. The effectiveness of vinflunine treatment was comparable with an overall response rate of 22.2% and 23.7% respectively and a median progression-free survival of 2.76 months in both groups. Median overall survival was 5.0 months in UTUC compared to 8.2 months in the LTUC group (p=0.478). The safety profile was in accordance with previous vinflunine experiences, with a comparable frequency of adverse events in both groups.
CONCLUSION: Vinflunine can be applied in the 2nd line for UC regardless of the primary tumor localization.
PATIENTS AND METHODS: A post-hoc subgroup analysis of a non-interventional study investigating vinflunine after failure of a platinum-based chemotherapy in metastatic/locally advanced UC patients was performed.
RESULTS: A total of 18 and 59 out of 77 patients had UTUC and LTUC, respectively. The effectiveness of vinflunine treatment was comparable with an overall response rate of 22.2% and 23.7% respectively and a median progression-free survival of 2.76 months in both groups. Median overall survival was 5.0 months in UTUC compared to 8.2 months in the LTUC group (p=0.478). The safety profile was in accordance with previous vinflunine experiences, with a comparable frequency of adverse events in both groups.
CONCLUSION: Vinflunine can be applied in the 2nd line for UC regardless of the primary tumor localization.
Hong MH, Kim CG, Koh YW, et al.
Efficacy and safety of vinorelbine plus cisplatin chemotherapy for patients with recurrent and/or metastatic salivary gland cancer of the head and neck.
Head Neck. 2018; 40(1):55-62 [PubMed] Related Publications
Efficacy and safety of vinorelbine plus cisplatin chemotherapy for patients with recurrent and/or metastatic salivary gland cancer of the head and neck.
Head Neck. 2018; 40(1):55-62 [PubMed] Related Publications
BACKGROUND: The purpose of this study was to investigate the efficacy and safety of vinorelbine plus cisplatin chemotherapy in patients with recurrent and/or metastatic salivary gland cancer of the head and neck.
METHODS: In this single-arm phase II study, patients with recurrent and/or metastatic salivary gland cancer were treated with i.v. vinorelbine (25 mg/m
RESULTS: Between September 2008 and November 2014, 40 patients with recurrent and/or metastatic salivary gland cancer received vinorelbine plus cisplatin chemotherapy. The objective response rate was 35.0%, including 1 complete response. Median PFS and OS rates were 6.3 months and 16.9 months, respectively. No treatment-related deaths occurred.
CONCLUSION: Administering vinorelbine plus cisplatin chemotherapy to patients with recurrent and/or metastatic salivary gland cancers is safe and effective.
METHODS: In this single-arm phase II study, patients with recurrent and/or metastatic salivary gland cancer were treated with i.v. vinorelbine (25 mg/m
RESULTS: Between September 2008 and November 2014, 40 patients with recurrent and/or metastatic salivary gland cancer received vinorelbine plus cisplatin chemotherapy. The objective response rate was 35.0%, including 1 complete response. Median PFS and OS rates were 6.3 months and 16.9 months, respectively. No treatment-related deaths occurred.
CONCLUSION: Administering vinorelbine plus cisplatin chemotherapy to patients with recurrent and/or metastatic salivary gland cancers is safe and effective.
Alotaibi AAA, Najafzadeh M, Davies JD, et al.
Inhibition of survivin expression after using oxaliplatin and vinflunine to induce cytogenetic damage in vitro in lymphocytes from colon cancer patients and healthy individuals.
Mutagenesis. 2017; 32(5):517-524 [PubMed] Related Publications
Inhibition of survivin expression after using oxaliplatin and vinflunine to induce cytogenetic damage in vitro in lymphocytes from colon cancer patients and healthy individuals.
Mutagenesis. 2017; 32(5):517-524 [PubMed] Related Publications
Chemotherapy drugs usually inflict a lethal dose to tumour cells with the consequence that these cells are being killed by cell death. However, each round of chemotherapy also causes damage to normal somatic cells. The DNA cross-linking agent oxaliplatin (OXP), which causes DNA double-strand breaks, and vinflunine (VFN), which disrupts the mitotic spindle, are two of these chemotherapy drugs which were evaluated in vitro using peripheral lymphocytes from colorectal cancer patients and healthy individuals to determine any differential response. Endpoints examined included micronucleus (MN) induction using the cytokinesis-blocked micronucleus (CBMN) assay and pancentromeric fluorescence in situ hybridisation. Also, survivin expression was monitored since it regulates the mitotic spindle checkpoint and inhibits apoptosis. OXP produced cytogenetic damage (micronuclei in binucleated cells) via its clastogenic but also previously unknown aneugenic action, possibly through interfering with topoisomerase II, whilst VFN produced micronuclei in mononucleated cells because of incomplete karyokinesis. Survivin expression was found to be significantly reduced in a concentration-dependent manner by not only OXP but surprisingly also VFN. This resulted in large numbers of multinucleated cells found with the CBMN assay. As survivin is upregulated in cancers, eliminating apoptosis inhibition might provide a more targeted chemotherapy approach; particularly, when considering VFN, which only affects cycling cells by inhibiting their mitotic spindle, and alongside possibly other pro-apoptotic compounds. Hence, these newly found properties of VFN -the inhibition of survivin expression-might demonstrate a promising chemotherapeutic approach as VFN induces less DNA damage in normal somatic cells compared to other chemotherapeutic compounds.
He K, Wang X, Guan X, et al.
Vinorelbine Plus Gemcitabine or Cisplatin as First-line Treatment of HER2-negative Advanced Breast Cancer.
Anticancer Res. 2017; 37(10):5647-5653 [PubMed] Related Publications
Vinorelbine Plus Gemcitabine or Cisplatin as First-line Treatment of HER2-negative Advanced Breast Cancer.
Anticancer Res. 2017; 37(10):5647-5653 [PubMed] Related Publications
AIM: To evaluate the efficacy and toxicity of vinorelbine and gemcitabine (NG) versus vinorelbine and cisplatin (NP) in anthracycline- and taxane-pretreated patients with HER2-negative advanced breast cancer.
PATIENTS AND METHODS: Patients were randomly assigned on a 1:1 schedule to receive no more than six cycles of NG or NP. Dosing for the NG group was 25 mg/m
RESULTS: The full analysis set comprised of 37 patients receiving NG and 37 receiving NP. The DCR was 70.3% with NG and 64.9% with NP (p=0.619). Median PFS were 7 months (95% CI=5.88-8.12) and 6 months (95% CI=5.29-6.71) respectively in NG and NP group [hazard ratio (HR)=1.696; 95% confidence interval (CI)=0.73-3.93; p=0.217)]. Corresponding median OS was 18 (95% CI=10.35-13.65) months and 12 (95% CI=15.83-20.17) months (HR=1.219; 95% CI=0.67-2.23; p=0.521). For adverse events, neutropenia and nausea/vomiting were milder in the NG group than in the NP group (all p<0.05).
CONCLUSION: Although no significant differences were observed in terms of DCR, PFS and OS, with milder toxicity, NG appeared to be a more valuable first-line treatment regimen than NP in anthracycline- and taxane-pretreated patients with HER2-negative advanced breast cancer.
PATIENTS AND METHODS: Patients were randomly assigned on a 1:1 schedule to receive no more than six cycles of NG or NP. Dosing for the NG group was 25 mg/m
RESULTS: The full analysis set comprised of 37 patients receiving NG and 37 receiving NP. The DCR was 70.3% with NG and 64.9% with NP (p=0.619). Median PFS were 7 months (95% CI=5.88-8.12) and 6 months (95% CI=5.29-6.71) respectively in NG and NP group [hazard ratio (HR)=1.696; 95% confidence interval (CI)=0.73-3.93; p=0.217)]. Corresponding median OS was 18 (95% CI=10.35-13.65) months and 12 (95% CI=15.83-20.17) months (HR=1.219; 95% CI=0.67-2.23; p=0.521). For adverse events, neutropenia and nausea/vomiting were milder in the NG group than in the NP group (all p<0.05).
CONCLUSION: Although no significant differences were observed in terms of DCR, PFS and OS, with milder toxicity, NG appeared to be a more valuable first-line treatment regimen than NP in anthracycline- and taxane-pretreated patients with HER2-negative advanced breast cancer.
Wang J, Zheng R, Wang Z, et al.
Efficacy and Safety of Vinorelbine Plus Cisplatin vs. Gemcitabine Plus Cisplatin for Treatment of Metastatic Triple-Negative Breast Cancer After Failure with Anthracyclines and Taxanes.
Med Sci Monit. 2017; 23:4657-4664 [PubMed] Free Access to Full Article Related Publications
Efficacy and Safety of Vinorelbine Plus Cisplatin vs. Gemcitabine Plus Cisplatin for Treatment of Metastatic Triple-Negative Breast Cancer After Failure with Anthracyclines and Taxanes.
Med Sci Monit. 2017; 23:4657-4664 [PubMed] Free Access to Full Article Related Publications
BACKGROUND This study aimed to compare the efficacy and safety of vinorelbine plus cisplatin (NP regimen) vs. gemcitabine plus cisplatin (GP regimen) for treatment of metastatic TNBC after failure with anthracyclines and taxanes. MATERIAL AND METHODS A total of 48 patients with metastatic TNBC that failed in anthracyclines and taxanes treatment were enrolled and randomly grouped. Patients in the NP group (n=22) were given 25 mg/m² vinorelbine on days 1 and 8 and 25 mg/m² cisplatin on days 2-4 of each 21-day cycle, while subjects in the GP group (n=26) were administered 1000 mg/m² gemcitabine on days 1 and 8 and 25 mg/m² cisplatin on days 2-4 of each 21-day cycle. The treatment response and adverse events were compared between the 2 groups every 2 cycles. RESULTS The ORR, DCR, and median TTP were 45.5%, 77.3%, and 5 months in the NP group, and 46.2%, 80.8%, and 5.2 months in the GP group, and no significant differences were observed in ORR, DCR, and median TTP between the 2 groups (P>0.05). The major adverse events included grade I-II bone marrow inhibition, gastrointestinal reactions, and phlebitis, and a lower incidence of thrombocytopenia and rash and a higher incidence of phlebitis was found in the NP group than in the GP group (P<0.05). CONCLUSIONS Either NP or GP regimen is active and tolerated in treatment of metastatic TNBC with anthracyclines and/or taxanes resistance, which may be used as a salvage treatment for metastatic TNBC.
Lukesh JC, Carney DW, Dong H, et al.
Vinblastine 20' Amides: Synthetic Analogues That Maintain or Improve Potency and Simultaneously Overcome Pgp-Derived Efflux and Resistance.
J Med Chem. 2017; 60(17):7591-7604 [PubMed] Free Access to Full Article Related Publications
Vinblastine 20' Amides: Synthetic Analogues That Maintain or Improve Potency and Simultaneously Overcome Pgp-Derived Efflux and Resistance.
J Med Chem. 2017; 60(17):7591-7604 [PubMed] Free Access to Full Article Related Publications
A series of 180 vinblastine 20' amides were prepared in three steps from commercially available starting materials, systematically exploring a typically inaccessible site in the molecule enlisting a powerful functionalization strategy. Clear structure-activity relationships and a structural model were developed in the studies which provided many such 20' amides that exhibit substantial and some even remarkable enhancements in potency, many that exhibit further improvements in activity against a Pgp overexpressing resistant cancer cell line, and an important subset of the vinblastine analogues that display little or no differential in activity against a matched pair of vinblastine sensitive and resistant (Pgp overexpressing) cell lines. The improvements in potency directly correlated with target tubulin binding affinity, and the reduction in differential functional activity against the sensitive and Pgp overexpressing resistant cell lines was found to correlate directly with an impact on Pgp-derived efflux.
Kipper FC, Silva AO, Marc AL, et al.
Vinblastine and antihelmintic mebendazole potentiate temozolomide in resistant gliomas.
Invest New Drugs. 2018; 36(2):323-331 [PubMed] Related Publications
Vinblastine and antihelmintic mebendazole potentiate temozolomide in resistant gliomas.
Invest New Drugs. 2018; 36(2):323-331 [PubMed] Related Publications
Glioblastoma (GBM) is a very aggressive tumor that has not had substantial therapeutic improvement since the introduction of temozolomide (TMZ) in combination with radiotherapy. Combining TMZ with other chemotherapeutic agents is a strategy that could be further explored for GBM. To search for molecular predictors of TMZ resistance, the TCGA (The Cancer Genome Atlas) database was utilized to assess the impact of specific genes on TMZ response. Patients whose tumors expressed low levels of FGFR3 and AKT2 responded poorly to TMZ. Combination treatment of vinblastine (VBL) plus mebendazole (MBZ) with TMZ was more effective in reducing cell number in most cultures when compared to TMZ alone, especially in cells with low expression levels of FGFR3 and AKT2. Cell cycle distribution and nuclear morphometric analysis indicated that the triple combination of TMZ, VBL and MBZ (TVM) was able to induce polyploidy and senescence, in addition to increasing the Notch3 RNA level in patient-derived gliomas. Thus, this set of data suggests that the triple combination of TMZ, VBL and MBZ may be a considerable therapeutic alternative for the TMZ-tolerant gliomas that harbor low expression of FGFR3/AKT2.
Chen M, Lei X, Shi C, et al.
Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents.
J Clin Invest. 2017; 127(10):3689-3701 [PubMed] Free Access to Full Article Related Publications
Pericyte-targeting prodrug overcomes tumor resistance to vascular disrupting agents.
J Clin Invest. 2017; 127(10):3689-3701 [PubMed] Free Access to Full Article Related Publications
Blood vessels in the tumor periphery have high pericyte coverage and are resistant to vascular disrupting agents (VDAs). VDA treatment resistance leads to a viable peripheral tumor rim that contributes to treatment failure and disease recurrence. Here, we provide evidence to support a hypothesis that shifting the target of VDAs from tumor vessel endothelial cells to pericytes disrupts tumor peripheral vessels and the viable rim, circumventing VDA treatment resistance. Through chemical engineering, we developed Z-GP-DAVLBH (from the tubulin-binding VDA desacetylvinblastine monohydrazide [DAVLBH]) as a prodrug that can be selectively activated by fibroblast activation protein α (FAPα) in tumor pericytes. Z-GP-DAVLBH selectively destroys the cytoskeleton of FAPα-expressing tumor pericytes, disrupting blood vessels both within the core and around the periphery of tumors. As a result, Z-GP-DAVLBH treatment eradicated the otherwise VDA-resistant tumor rim and led to complete regression of tumors in multiple lines of xenografts without producing the drug-related toxicity that is associated with similar doses of DAVLBH. This study demonstrates that targeting tumor pericytes with an FAPα-activated VDA prodrug represents a potential vascular disruption strategy in overcoming tumor resistance to VDA treatments.
Kenmotsu H, Ohde Y, Wakuda K, et al.
Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer.
Cancer Chemother Pharmacol. 2017; 80(3):609-614 [PubMed] Related Publications
Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer.
Cancer Chemother Pharmacol. 2017; 80(3):609-614 [PubMed] Related Publications
PURPOSE: Despite the efficacy of postoperative adjuvant cisplatin (CDDP)-based chemotherapy for patients who have undergone surgical resection of non-small cell lung cancer (NSCLC), few reports have presented survival data for Asian patients treated with adjuvant chemotherapy involving a combination of CDDP and vinorelbine (VNR). This study was performed to evaluate the survival of patients with NSCLC who received postoperative adjuvant chemotherapy comprising CDDP + VNR.
METHODS: We retrospectively evaluated patients with NSCLC who received adjuvant chemotherapy comprising CDDP + VNR at the Shizuoka Cancer Center between February 2006 and October 2011.
RESULTS: One hundred patients who underwent surgical resection of NSCLC were included in this study. The patients' characteristics were as follows: median age 63 years (range 36-74 years), female 34%, never-smokers 20%, and non-squamous NSCLC 73%. Pathological stages IIA, IIB, and IIIA were observed in 31, 22, and 47% of patients, respectively. The 5- and 2-year overall survival rates were 73 and 93%, respectively. The 5- and 2-year relapse-free survival rates were 53 and 62%, respectively. Univariate analysis of prognostic factors showed that patient characteristics (sex, histology, and pathological stage) and CDDP dose intensity were not significantly associated with survival. In 48 patients who developed NSCLC recurrence, the 5-year survival rate after recurrence was 29%, and the median survival time after recurrence was 37 months.
CONCLUSIONS: Our results suggest that the prognosis after surgical resection of NSCLC and adjuvant chemotherapy comprising CDDP + VNR might be improving compared with previous survival data of adjuvant chemotherapy for NSCLC.
METHODS: We retrospectively evaluated patients with NSCLC who received adjuvant chemotherapy comprising CDDP + VNR at the Shizuoka Cancer Center between February 2006 and October 2011.
RESULTS: One hundred patients who underwent surgical resection of NSCLC were included in this study. The patients' characteristics were as follows: median age 63 years (range 36-74 years), female 34%, never-smokers 20%, and non-squamous NSCLC 73%. Pathological stages IIA, IIB, and IIIA were observed in 31, 22, and 47% of patients, respectively. The 5- and 2-year overall survival rates were 73 and 93%, respectively. The 5- and 2-year relapse-free survival rates were 53 and 62%, respectively. Univariate analysis of prognostic factors showed that patient characteristics (sex, histology, and pathological stage) and CDDP dose intensity were not significantly associated with survival. In 48 patients who developed NSCLC recurrence, the 5-year survival rate after recurrence was 29%, and the median survival time after recurrence was 37 months.
CONCLUSIONS: Our results suggest that the prognosis after surgical resection of NSCLC and adjuvant chemotherapy comprising CDDP + VNR might be improving compared with previous survival data of adjuvant chemotherapy for NSCLC.
Kolek V, Grygárková I, Koubková L, et al.
Carboplatin with intravenous and subsequent oral administration of vinorelbine in resected non-small-cell-lung cancer in real-world set-up.
PLoS One. 2017; 12(7):e0181803 [PubMed] Free Access to Full Article Related Publications
Carboplatin with intravenous and subsequent oral administration of vinorelbine in resected non-small-cell-lung cancer in real-world set-up.
PLoS One. 2017; 12(7):e0181803 [PubMed] Free Access to Full Article Related Publications
OBJECTIVES: Adjuvant cisplatin-based chemotherapy is recommended for routine use in patients with Stage IIA, IIB or IIIA non-small cell lung cancer (NSCLC) after complete resection. Results obtained for Stage IB were not conclusive. While vinorelbine plus cisplatin is the preferred choice after resection, combining vinorelbine with carboplatin promises improved compliance and delivery of drugs due to lower toxicity. We evaluated the impact of this option on treatment compliance and survival under real-world conditions.
MATERIAL AND METHODS: A prospective, single-arm, multicenter, non-interventional study evaluated the tolerability, dose intensity and survival resulting from adjuvant use of intravenous carboplatin (AUC 5 on day 1) with vinorelbine administered both intravenously (25 mg/m2 on day 1) and orally (60 mg/m2 on day 8) within four cycles of 21 days each. A total of 74 patients with a median age of 64 years were observed.
RESULTS: The mean number of accomplished cycles was 3.78, and 62 patients (83.7%) completed all four planned cycles. Relative dose intensity for carboplatin was 88.9%, for intravenous vinorelbine 93.1%, and for oral vinorelbine 83.2%. Median follow-up was 4.73 years. Median disease-specific survival (DSS) was 7.63 years, median overall survival (OS) was 5.90 years, median disease-free survival (DFS0) was 4.43 years, and five-year survival was 56.2%. TNM stage of disease significantly affected DSS and OS. Favorable survival was observed in females, nonsmokers, patients aged over 65 years, patient with prior lobectomy, patients with tumor of squamous histology, and those who finished the planned therapy, but the differences were non-significant.
CONCLUSION: Adjuvant carboplatin with vinorelbine switched from intravenous to oral administration was shown to be a favorable regimen with regard to tolerability and safety. Compliance to therapy was high, and survival parameters were promising, showing that applied regimen can be another potential option for adjuvant chemotherapy in patients with NSCLC.
MATERIAL AND METHODS: A prospective, single-arm, multicenter, non-interventional study evaluated the tolerability, dose intensity and survival resulting from adjuvant use of intravenous carboplatin (AUC 5 on day 1) with vinorelbine administered both intravenously (25 mg/m2 on day 1) and orally (60 mg/m2 on day 8) within four cycles of 21 days each. A total of 74 patients with a median age of 64 years were observed.
RESULTS: The mean number of accomplished cycles was 3.78, and 62 patients (83.7%) completed all four planned cycles. Relative dose intensity for carboplatin was 88.9%, for intravenous vinorelbine 93.1%, and for oral vinorelbine 83.2%. Median follow-up was 4.73 years. Median disease-specific survival (DSS) was 7.63 years, median overall survival (OS) was 5.90 years, median disease-free survival (DFS0) was 4.43 years, and five-year survival was 56.2%. TNM stage of disease significantly affected DSS and OS. Favorable survival was observed in females, nonsmokers, patients aged over 65 years, patient with prior lobectomy, patients with tumor of squamous histology, and those who finished the planned therapy, but the differences were non-significant.
CONCLUSION: Adjuvant carboplatin with vinorelbine switched from intravenous to oral administration was shown to be a favorable regimen with regard to tolerability and safety. Compliance to therapy was high, and survival parameters were promising, showing that applied regimen can be another potential option for adjuvant chemotherapy in patients with NSCLC.
Passalacqua R, Lazzarelli S, Donini M, et al.
Real-life clinical practice results with vinflunine in patients with relapsed platinum-treated metastatic urothelial carcinoma: an Italian multicenter study (MOVIE-GOIRC 01-2014).
BMC Cancer. 2017; 17(1):493 [PubMed] Free Access to Full Article Related Publications
Real-life clinical practice results with vinflunine in patients with relapsed platinum-treated metastatic urothelial carcinoma: an Italian multicenter study (MOVIE-GOIRC 01-2014).
BMC Cancer. 2017; 17(1):493 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Vinflunine is the only chemotherapeutic agent shown to improve survival in platinum-refractory patients with metastatic transitional cell carcinoma of the urothelium (TCCU) in a phase III clinical trial, which led to product registration for this indication in Europe. The aim of this study was to assess the efficacy of vinflunine and to evaluate the prognostic significance of risk factors in a large, unselected cohort of patients with metastatic TCCU treated according to routine clinical practice.
METHODS: This was a retrospective multicenter study. Italian cancer centers were selected if, according to the Registry of the Italian Medicines Agency (AIFA), at least four patients had been treated with vinflunine between February 2011 and June 2014, after first- or second-line platinum-based chemotherapy. The primary objective was to test whether the efficacy measured by overall survival (OS) in the registration study could be confirmed in routine clinical practice. Multivariate analysis was carried out using Cox proportional hazard model.
RESULTS: A total of 217 patients were treated in 28 Italian centers. Median age was 69 years (IQR 62-76) and 84% were male; Eastern Cooperative Oncology Group performance status (ECOG PS) was ≥ 1 in 53% of patients. The median number of cycles was 4 (IQR 2-6); 29%, 35%, and 36% received an initial dose of 320 mg/m
CONCLUSIONS: In routine clinical practice the results obtained with VFL seem to be better than the results of the registration trial and reinforce evidence supporting its use after failure of a platinum-based chemotherapy.
METHODS: This was a retrospective multicenter study. Italian cancer centers were selected if, according to the Registry of the Italian Medicines Agency (AIFA), at least four patients had been treated with vinflunine between February 2011 and June 2014, after first- or second-line platinum-based chemotherapy. The primary objective was to test whether the efficacy measured by overall survival (OS) in the registration study could be confirmed in routine clinical practice. Multivariate analysis was carried out using Cox proportional hazard model.
RESULTS: A total of 217 patients were treated in 28 Italian centers. Median age was 69 years (IQR 62-76) and 84% were male; Eastern Cooperative Oncology Group performance status (ECOG PS) was ≥ 1 in 53% of patients. The median number of cycles was 4 (IQR 2-6); 29%, 35%, and 36% received an initial dose of 320 mg/m
CONCLUSIONS: In routine clinical practice the results obtained with VFL seem to be better than the results of the registration trial and reinforce evidence supporting its use after failure of a platinum-based chemotherapy.
Mu LM, Bu YZ, Liu L, et al.
Lipid vesicles containing transferrin receptor binding peptide TfR-T
Sci Rep. 2017; 7(1):3487 [PubMed] Free Access to Full Article Related Publications
Lipid vesicles containing transferrin receptor binding peptide TfR-T
Sci Rep. 2017; 7(1):3487 [PubMed] Free Access to Full Article Related Publications
Surgery and radiotherapy cannot fully remove brain glioma; thus, chemotherapy continues to play an important role in treatment of this illness. However, because of the restriction of the blood-brain barrier (BBB) and the regeneration of glioma stem cells, post-chemotherapy relapse usually occurs. Here, we report a potential solution to these issues that involves a type of novel multifunctional vinblastine liposomes equipped with transferrin receptor binding peptide TfR-T
Yamamura J, Masuda N, Yamamoto D, et al.
Gemcitabine and Vinorelbine Combination Chemotherapy in Taxane-Pretreated Patients with Metastatic Breast Cancer: A Phase II Study of the Kinki Multidisciplinary Breast Oncology Group (KMBOG) 1015.
Chemotherapy. 2017; 62(5):307-313 [PubMed] Related Publications
Gemcitabine and Vinorelbine Combination Chemotherapy in Taxane-Pretreated Patients with Metastatic Breast Cancer: A Phase II Study of the Kinki Multidisciplinary Breast Oncology Group (KMBOG) 1015.
Chemotherapy. 2017; 62(5):307-313 [PubMed] Related Publications
BACKGROUND: This phase II study was conducted to evaluate the efficacy and safety of the chemotherapy combination of gemcitabine and vinorelbine in taxane-pretreated Japanese metastatic breast cancer patients.
METHODS: In this multicenter, phase II, single-arm study, patients with recurrent or metastatic HER2-negative breast cancer were administered gemcitabine (1,200 mg/m2) and vinorelbine (25 mg/m2) intravenously on days 1 and 8 every 3 weeks. The primary endpoint was the objective response rate, and other endpoints included progression-free survival, overall survival, and safety.
RESULTS: A total of 42 patients were enrolled in this study. The objective response rate and clinical benefit rate were 24 and 43%, respectively. The median progression-free survival was 4.0 months. The median overall survival was 11.1 months. Grade 3/4 neutropenia was the most common hematologic toxicity, occurring in 22 patients (54%). Nonhematologic toxicity was moderate and transient, with fatigue (48%) being the most common condition and no severe adverse event reported.
CONCLUSION: The combination of gemcitabine and vinorelbine is an effective and tolerable regimen for HER2-negative, taxane-pretreated, metastatic breast cancer patients in Japan.
METHODS: In this multicenter, phase II, single-arm study, patients with recurrent or metastatic HER2-negative breast cancer were administered gemcitabine (1,200 mg/m2) and vinorelbine (25 mg/m2) intravenously on days 1 and 8 every 3 weeks. The primary endpoint was the objective response rate, and other endpoints included progression-free survival, overall survival, and safety.
RESULTS: A total of 42 patients were enrolled in this study. The objective response rate and clinical benefit rate were 24 and 43%, respectively. The median progression-free survival was 4.0 months. The median overall survival was 11.1 months. Grade 3/4 neutropenia was the most common hematologic toxicity, occurring in 22 patients (54%). Nonhematologic toxicity was moderate and transient, with fatigue (48%) being the most common condition and no severe adverse event reported.
CONCLUSION: The combination of gemcitabine and vinorelbine is an effective and tolerable regimen for HER2-negative, taxane-pretreated, metastatic breast cancer patients in Japan.
Andersson M, López-Vega JM, Petit T, et al.
Efficacy and Safety of Pertuzumab and Trastuzumab Administered in a Single Infusion Bag, Followed by Vinorelbine: VELVET Cohort 2 Final Results.
Oncologist. 2017; 22(10):1160-1168 [PubMed] Free Access to Full Article Related Publications
Efficacy and Safety of Pertuzumab and Trastuzumab Administered in a Single Infusion Bag, Followed by Vinorelbine: VELVET Cohort 2 Final Results.
Oncologist. 2017; 22(10):1160-1168 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: VELVET Cohort 1 demonstrated the applicability of pertuzumab, trastuzumab, and vinorelbine as an alternative first-line treatment regimen for patients with HER2-positive locally advanced or metastatic breast cancer (MBC) who cannot receive docetaxel. Co-infusion of pertuzumab and trastuzumab may reduce clinic time and medical resource utilization. We report results from Cohort 2, in which pertuzumab and trastuzumab were co-infused, followed by vinorelbine.
PATIENTS AND METHODS: During cycle 1, patients with HER2-positive locally advanced or MBC received loading doses of pertuzumab (840 mg) and trastuzumab (8 mg/kg) on consecutive days, followed by vinorelbine (25 mg/m
RESULTS: Cohort 2 enrolled 107 patients. The ORR was 63.7% (95% confidence interval [CI] 53.0-73.6) in patients with measurable disease (91/107; 85.0%). Median PFS was 11.5 months (95% CI 10.3-15.8). The most common adverse events [AEs] were diarrhea (57.9%), neutropenia (57.0%), and nausea (41.1%). Grade ≥3 AEs occurred in 85 patients (79.4%) and serious AEs in 44 patients (41.1%). Eighteen patients (16.8%) had AEs suggestive of congestive heart failure.
CONCLUSION: These results support the feasibility of pertuzumab and trastuzumab co-infusion from a safety perspective and support Cohort 1 conclusions that vinorelbine offers an alternative chemotherapy companion for pertuzumab and trastuzumab.
PATIENTS AND METHODS: During cycle 1, patients with HER2-positive locally advanced or MBC received loading doses of pertuzumab (840 mg) and trastuzumab (8 mg/kg) on consecutive days, followed by vinorelbine (25 mg/m
RESULTS: Cohort 2 enrolled 107 patients. The ORR was 63.7% (95% confidence interval [CI] 53.0-73.6) in patients with measurable disease (91/107; 85.0%). Median PFS was 11.5 months (95% CI 10.3-15.8). The most common adverse events [AEs] were diarrhea (57.9%), neutropenia (57.0%), and nausea (41.1%). Grade ≥3 AEs occurred in 85 patients (79.4%) and serious AEs in 44 patients (41.1%). Eighteen patients (16.8%) had AEs suggestive of congestive heart failure.
CONCLUSION: These results support the feasibility of pertuzumab and trastuzumab co-infusion from a safety perspective and support Cohort 1 conclusions that vinorelbine offers an alternative chemotherapy companion for pertuzumab and trastuzumab.


 Cancer Prevention and Risk Reduction
Cancer Prevention and Risk Reduction