Found this page useful?
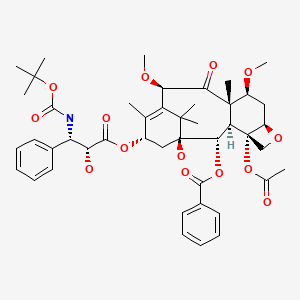
Cabazitaxel
 Web Resources: Cabazitaxel
Web Resources: Cabazitaxel Recent Research Publications
Recent Research PublicationsWeb Resources: Cabazitaxel (5 links)
Cancer Research UK
Macmillan Cancer Support
NHS Evidence
MedlinePlus
PubChem
Recent Research Publications
Shiota M, Nakamura M, Yokomizo A, et al.
Therapeutic Outcome of >10 Cycles of Cabazitaxel for Castration-resistant Prostate Cancer: A Multi-institutional Study.
Anticancer Res. 2019; 39(8):4411-4414 [PubMed] Related Publications
Therapeutic Outcome of >10 Cycles of Cabazitaxel for Castration-resistant Prostate Cancer: A Multi-institutional Study.
Anticancer Res. 2019; 39(8):4411-4414 [PubMed] Related Publications
BACKGROUND/AIM: Cabazitaxel use has usually been limited to up to 10 cycles in most countries according to the protocol in the TROPIC trial. Therefore, clinical data on cabazitaxel use beyond 10 cycles is limited. The aim of this study was to report the therapeutic outcome of cabazitaxel chemotherapy administered for >10 cycles.
PATIENTS AND METHODS: This study included 74 Japanese patients with prostate cancer between 2014 and 2017. Patients background, and treatment outcomes including PSA decline, progression-free survival, treatment-failure-free survival, overall survival, and adverse events were investigated, comparing patients treated with ≤10 and >10 cycles.
RESULTS: Patients characteristics were favorable as indicated by the higher number of cycles of prior docetaxel chemotherapy, absence of pain, and absence of bony and visceral metastases among men who received >10 cycles of cabazitaxel. PSA response, progression-free survival, treatment-failure-free survival and overall survival were better among patients treated with >10 cycles of cabazitaxel compared to those treated with ≤10 cycles. The incidence of severe adverse events was similar between the two groups.
CONCLUSION: Taken together, this study suggested that continuous chemotherapy with cabazitaxel beyond 10 cycles may be beneficial.
PATIENTS AND METHODS: This study included 74 Japanese patients with prostate cancer between 2014 and 2017. Patients background, and treatment outcomes including PSA decline, progression-free survival, treatment-failure-free survival, overall survival, and adverse events were investigated, comparing patients treated with ≤10 and >10 cycles.
RESULTS: Patients characteristics were favorable as indicated by the higher number of cycles of prior docetaxel chemotherapy, absence of pain, and absence of bony and visceral metastases among men who received >10 cycles of cabazitaxel. PSA response, progression-free survival, treatment-failure-free survival and overall survival were better among patients treated with >10 cycles of cabazitaxel compared to those treated with ≤10 cycles. The incidence of severe adverse events was similar between the two groups.
CONCLUSION: Taken together, this study suggested that continuous chemotherapy with cabazitaxel beyond 10 cycles may be beneficial.
Roviello G, Corona SP, Conca R, et al.
Is there still a place for vinorelbine in advanced metastatic castration resistant prostate cancer?
Medicine (Baltimore). 2019; 98(26):e16249 [PubMed] Free Access to Full Article Related Publications
Is there still a place for vinorelbine in advanced metastatic castration resistant prostate cancer?
Medicine (Baltimore). 2019; 98(26):e16249 [PubMed] Free Access to Full Article Related Publications
The aim of this paper was to evaluate the activity and tolerability of oral vinorelbine in patients with advanced castration resistant prostate cancer (CRPC) who progressed after a minimum of three lines including: abiraterone acetate, docetaxel, cabazitaxel, and enzalutamide.Treatment consisted of weekly oral vinorelbine 60 mg/m. Chemotherapy was administered until disease progression or unacceptable toxicity.Twenty-six patients received vinorelbine: their median age was 74 years (range 58-84 years). Twenty-four (92.3%) patients had bone metastases. A decrease in PSA levels ≥50% was observed in 2 patients (7.7%). Among the subjects who were symptomatic at baseline, pain was reduced in 3 patients (13.6%) with a significant decrease in analgesic use. Median progression-free survival was 9 weeks (95% CI: 7 to 11) and median overall survival was 17 weeks (95% CI: 12 to 22). Treatment was well tolerated, and no grade 4 toxicities were observed.Our findings do not suggest the use of oral vinorelbine on a weekly schedule, in CRPC heavily pre-treated.
Ito T, Kanao K, Takahara K, et al.
Optimal Timing of Cabazitaxel Introduction for Japanese Patients With Metastatic Castration-resistant Prostate Cancer.
Anticancer Res. 2019; 39(6):3089-3094 [PubMed] Related Publications
Optimal Timing of Cabazitaxel Introduction for Japanese Patients With Metastatic Castration-resistant Prostate Cancer.
Anticancer Res. 2019; 39(6):3089-3094 [PubMed] Related Publications
BACKGROUND/AIM: Limited information is available to help physicians decide when to introduce cabazitaxel for metastatic castration-resistant prostate cancer (mCRPC) patients. The objective of this study was to assess the optimal timing of cabazitaxel introduction.
PATIENTS AND METHODS: The clinical outcomes of 66 mCRPC patients receiving cabazitaxel following failure of docetaxel were retrospectively analyzed.
RESULTS: Among the parameters possibly affecting the timing of cabazitaxel introduction, only an increased prostate-specific antigen (PSA) value from the diagnosis of CRPC had a significant impact on overall survival (OS) after the introduction of cabazitaxel. Furthermore, there was a significant correlation between the increased PSA value from the diagnosis of CRPC and the baseline PSA value at cabazitaxel introduction. Multivariate analysis showed that only the baseline PSA value at cabazitaxel introduction is an independent predictor of OS.
CONCLUSION: A comparatively low PSA value could be an alternative index suggesting the optimal timing for cabazitaxel introduction.
PATIENTS AND METHODS: The clinical outcomes of 66 mCRPC patients receiving cabazitaxel following failure of docetaxel were retrospectively analyzed.
RESULTS: Among the parameters possibly affecting the timing of cabazitaxel introduction, only an increased prostate-specific antigen (PSA) value from the diagnosis of CRPC had a significant impact on overall survival (OS) after the introduction of cabazitaxel. Furthermore, there was a significant correlation between the increased PSA value from the diagnosis of CRPC and the baseline PSA value at cabazitaxel introduction. Multivariate analysis showed that only the baseline PSA value at cabazitaxel introduction is an independent predictor of OS.
CONCLUSION: A comparatively low PSA value could be an alternative index suggesting the optimal timing for cabazitaxel introduction.
Drenberg CD, Shelat A, Dang J, et al.
A high-throughput screen indicates gemcitabine and JAK inhibitors may be useful for treating pediatric AML.
Nat Commun. 2019; 10(1):2189 [PubMed] Free Access to Full Article Related Publications
A high-throughput screen indicates gemcitabine and JAK inhibitors may be useful for treating pediatric AML.
Nat Commun. 2019; 10(1):2189 [PubMed] Free Access to Full Article Related Publications
Improvement in survival has been achieved for children and adolescents with AML but is largely attributed to enhanced supportive care as opposed to the development of better treatment regimens. High risk subtypes continue to have poor outcomes with event free survival rates <40% despite the use of high intensity chemotherapy in combination with hematopoietic stem cell transplant. Here we combine high-throughput screening, intracellular accumulation assays, and in vivo efficacy studies to identify therapeutic strategies for pediatric AML. We report therapeutics not currently used to treat AML, gemcitabine and cabazitaxel, have broad anti-leukemic activity across subtypes and are more effective relative to the AML standard of care, cytarabine, both in vitro and in vivo. JAK inhibitors are selective for acute megakaryoblastic leukemia and significantly prolong survival in multiple preclinical models. Our approach provides advances in the development of treatment strategies for pediatric AML.
Anselmo da Costa I, Stenzl A, Bedke J
Inmunotherapy in prostate cancer.
Arch Esp Urol. 2019; 72(2):211-222 [PubMed] Related Publications
Inmunotherapy in prostate cancer.
Arch Esp Urol. 2019; 72(2):211-222 [PubMed] Related Publications
Prostate cancer (PCa) is the second most commonly diagnosed cancer. Although systemic chemotherapeutic agents, such as cabazitaxel, abiraterone and enzalutamde have become available to patients over the last decade, metastatic PCa is still an incurable disease. Immunotherapy is showing great promise in a wide range of other cancer types. To this day, the only immunotherapy approved by the FDA for PCa is the Sipuleucel-T vaccine, which showed significant clinical efficacy. Multiple clinical studies on immunotherapy in PCa are currently underway.
OBJECTIVES: Recent clinical trials have shown promising results in immunotherapeutic in treatment for PCa. The authors review previous clinical trials, as well as discuss and emphasize important emerging immunotherapies for PCa.
METHODS: Review of the published evidence related to immunotherapy in PCa. PubMed and clinicaltrials.gov databases were used to search for English papers and clinical trials.
RESULTS: Multiple clinical trials are testing different immunotherapeuticagents, as well as combinations thereof. The low grade of toxicity associated with these immunotherapies is an appealing advantage for patients, leading to an increased appreciation of theses types of treatments. Until now, only one clinical trial led to a new immunotherapeutic agent to be FDA approved. Importantphase II/III clinical trials are being conducted, and in the near future the concept of PCa treatment might be re-challenged.
CONCLUSIONS: Many trials are ongoing to determine the effects of immunotherapy in PCa. These studies may harvest important confirmatory data in the next years, with the potential to reshape PCa treatment.
OBJECTIVES: Recent clinical trials have shown promising results in immunotherapeutic in treatment for PCa. The authors review previous clinical trials, as well as discuss and emphasize important emerging immunotherapies for PCa.
METHODS: Review of the published evidence related to immunotherapy in PCa. PubMed and clinicaltrials.gov databases were used to search for English papers and clinical trials.
RESULTS: Multiple clinical trials are testing different immunotherapeuticagents, as well as combinations thereof. The low grade of toxicity associated with these immunotherapies is an appealing advantage for patients, leading to an increased appreciation of theses types of treatments. Until now, only one clinical trial led to a new immunotherapeutic agent to be FDA approved. Importantphase II/III clinical trials are being conducted, and in the near future the concept of PCa treatment might be re-challenged.
CONCLUSIONS: Many trials are ongoing to determine the effects of immunotherapy in PCa. These studies may harvest important confirmatory data in the next years, with the potential to reshape PCa treatment.
Wen L, Yao J, Valderrama A
Evaluation of Treatment Patterns and Costs in Patients with Prostate Cancer and Bone Metastases.
J Manag Care Spec Pharm. 2019; 25(3-b Suppl):S1-S11 [PubMed] Related Publications
Evaluation of Treatment Patterns and Costs in Patients with Prostate Cancer and Bone Metastases.
J Manag Care Spec Pharm. 2019; 25(3-b Suppl):S1-S11 [PubMed] Related Publications
BACKGROUND: There are a lack of guideline recommendations for patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing treatment progression and sequencing. Understanding treatment patterns and associated utilization and costs may help inform stakeholders and guide decision making.
OBJECTIVE: To describe treatment patterns and health care costs in prostate cancer (PC) patients with bone metastases treated with agents approved by the FDA for mCRPC.
METHODS: 2 large integrated claims databases (MarketScan and PharMetrics) were used to identify males aged ≥ 18 years who were diagnosed and treated for PC (ICD-9-CM code 185.xx or 233.4) with bone metastases (ICD-9-CM code 198.5) from June 2013 to September 2014. Patients were required to be continuously enrolled for ≥ 6 months before and after initiation of treatment with abiraterone, cabazitaxel, docetaxel, enzalutamide, mitoxantrone, radium-223, sipuleucel-T, or other chemotherapy. Study endpoints included lines of therapy, health care resource utilization per patient per month (PPPM), PPPM costs, and mortality rate. Descriptive analysis was completed for the study sample, and survival function was calculated via Kaplan-Meier estimates.
RESULTS: There were 953 patients meeting all inclusion criteria in the MarketScan database and 565 patients in the PharMetrics database. The median follow-up time was 18 months (interquartile range [IQR] = 14-23) for MarketScan and 14 months (IQR = 11-18) for PharMetrics. Mean age (SD) was 71 (± 10.7) and 66 (± 9.3) years, respectively. Before mCRPC treatment initiation, patients received palliative radiation therapy and bone antiresorptive therapy. For MarketScan and PharMetrics, respectively, 14.0% and 18.2% of patients received radiation therapy, 36.1% and 40.0% received denosumab; 16.5% and 16.8% received zoledronic acid; and 0.2% and 0.8% received pamidronate. Across both databases, abiraterone was the most commonly received bone metastasis treatment agent across all lines of therapy, except fourth line. Radium-223, cabazitaxel, and mitoxantrone were the least utilized therapies. The median cost PPPM during the post-index period was $10,916 (IQR=$5,334-$13,457) in MarketScan and $10,292 (IQR = $7,245-$14,699) in PharMetrics. The cost PPPM during the 6-month pre-index period was $2,643 (IQR = $850-$4,357) in MarketScan and $2,742 (IQR = $1,484-$4,730) in PharMetrics.
CONCLUSIONS: Patients were treated mainly with abiraterone across most lines of care, with radium-223, cabazitaxel, and mitoxantrone as the least utilized therapies. Median costs PPPM increased by approximately $8,900 after initiation of FDA-approved agents for mCRPC, with the largest increase in cost stemming from oral medications.
DISCLOSURES: Funding for this study was provided by Bayer HealthCare Pharmaceuticals. All authors were employees at Bayer HealthCare Pharmaceuticals at the time this study was conducted. This study was presented as a poster at the 2017 American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium; February 16-18, 2017; Orlando, FL.
OBJECTIVE: To describe treatment patterns and health care costs in prostate cancer (PC) patients with bone metastases treated with agents approved by the FDA for mCRPC.
METHODS: 2 large integrated claims databases (MarketScan and PharMetrics) were used to identify males aged ≥ 18 years who were diagnosed and treated for PC (ICD-9-CM code 185.xx or 233.4) with bone metastases (ICD-9-CM code 198.5) from June 2013 to September 2014. Patients were required to be continuously enrolled for ≥ 6 months before and after initiation of treatment with abiraterone, cabazitaxel, docetaxel, enzalutamide, mitoxantrone, radium-223, sipuleucel-T, or other chemotherapy. Study endpoints included lines of therapy, health care resource utilization per patient per month (PPPM), PPPM costs, and mortality rate. Descriptive analysis was completed for the study sample, and survival function was calculated via Kaplan-Meier estimates.
RESULTS: There were 953 patients meeting all inclusion criteria in the MarketScan database and 565 patients in the PharMetrics database. The median follow-up time was 18 months (interquartile range [IQR] = 14-23) for MarketScan and 14 months (IQR = 11-18) for PharMetrics. Mean age (SD) was 71 (± 10.7) and 66 (± 9.3) years, respectively. Before mCRPC treatment initiation, patients received palliative radiation therapy and bone antiresorptive therapy. For MarketScan and PharMetrics, respectively, 14.0% and 18.2% of patients received radiation therapy, 36.1% and 40.0% received denosumab; 16.5% and 16.8% received zoledronic acid; and 0.2% and 0.8% received pamidronate. Across both databases, abiraterone was the most commonly received bone metastasis treatment agent across all lines of therapy, except fourth line. Radium-223, cabazitaxel, and mitoxantrone were the least utilized therapies. The median cost PPPM during the post-index period was $10,916 (IQR=$5,334-$13,457) in MarketScan and $10,292 (IQR = $7,245-$14,699) in PharMetrics. The cost PPPM during the 6-month pre-index period was $2,643 (IQR = $850-$4,357) in MarketScan and $2,742 (IQR = $1,484-$4,730) in PharMetrics.
CONCLUSIONS: Patients were treated mainly with abiraterone across most lines of care, with radium-223, cabazitaxel, and mitoxantrone as the least utilized therapies. Median costs PPPM increased by approximately $8,900 after initiation of FDA-approved agents for mCRPC, with the largest increase in cost stemming from oral medications.
DISCLOSURES: Funding for this study was provided by Bayer HealthCare Pharmaceuticals. All authors were employees at Bayer HealthCare Pharmaceuticals at the time this study was conducted. This study was presented as a poster at the 2017 American Society of Clinical Oncology (ASCO) Genitourinary Cancers Symposium; February 16-18, 2017; Orlando, FL.
Shiota M, Nakamura M, Yokomizo A, et al.
Efficacy and safety of cabazitaxel for castration-resistant prostate cancer in patients with > 10 cycles of docetaxel chemotherapy: a multi-institutional study.
Med Oncol. 2019; 36(4):32 [PubMed] Related Publications
Efficacy and safety of cabazitaxel for castration-resistant prostate cancer in patients with > 10 cycles of docetaxel chemotherapy: a multi-institutional study.
Med Oncol. 2019; 36(4):32 [PubMed] Related Publications
This multi-institutional study aimed to investigate the efficacy and safety profiles of cabazitaxel after prior docetaxel chemotherapy in patients with castration-resistant prostate cancer (CRPC). This study included 63 Japanese patients with CRPC who were treated with cabazitaxel from 2014 to 2017. The oncological outcomes and adverse events (AEs) were documented, and prognostic factors for oncological outcomes and predictive factors for AEs were analysed. PSA decline was observed in 68.3% of patients, including 25.4% who achieved a ≥ 50% decline. The median progression-free survival, treatment failure-free survival, and overall survival were 4.3, 4.1, and 9.0 months, respectively. More cycles of prior docetaxel therapy was identified as common favourable prognostic factors for progression-free survival, treatment failure-free survival, and overall survival. Severe neutropenia, febrile neutropenia, and severe non-haematological AEs were observed in 73.0%, 33.3%, and 23.8% of patients, respectively. However, > 10 cycles of docetaxel was not associated with increased incidence of AEs. In conclusion, cabazitaxel chemotherapy was still active in Japanese CRPC patients treated with > 10 cycles of docetaxel chemotherapy, with an acceptable risk of AE burden. Treatment with cabazitaxel after > 10 cycles of docetaxel may be an appropriate option when it can be administered.
Terada N, Kamoto T, Tsukino H, et al.
The efficacy and toxicity of cabazitaxel for treatment of docetaxel-resistant prostate cancer correlating with the initial doses in Japanese patients.
BMC Cancer. 2019; 19(1):156 [PubMed] Free Access to Full Article Related Publications
The efficacy and toxicity of cabazitaxel for treatment of docetaxel-resistant prostate cancer correlating with the initial doses in Japanese patients.
BMC Cancer. 2019; 19(1):156 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: We analyzed the efficacy and toxicity of cabazitaxel (CBZ) at high and low initial doses in Japanese patients with docetaxel-resistant castration-resistant prostate cancer (CRPC).
METHODS: We retrospectively evaluated 118 patients who received CBZ for docetaxel-resistant CRPC in 10 university hospitals in Japan between 2014 and 2016. The rate of decrease of prostate-specific antigen (PSA), adverse events, progression-free survival (PFS), and overall survival (OS) were compared between patients receiving initially high (≥22.5 mg/m
RESULTS: PSA values decreased by > 50% in 22 patients (19%), with a higher frequency in the high-dose group than in the low-dose group (29 and 14%, P = 0.073). The median PFS time for the all-patient, high- and low-dose groups was 2.8 months (95% confidence interval [CI] 1.9-4.4), 2.1 months (1.2-5.5), and 3.0 months (2.0-4.4), respectively (P = 0.904). The median OS times were 16.3 months (95% CI 9.7-30.9), 30.9 months (11.8-47.4), and 10.2 months (8.6-20), respectively (P = 0.020). In multivariate analyses, PFS was significantly associated with existing bone metastasis at diagnosis (P = 0.005) and OS with PSA > 100 ng/ml (P = 0.007), hemoglobin < 12 g/dl (P = 0.030), and low initial CBZ dose (P = 0.030). Grade 4 neutropenia occurred in 53 patients (45%) and was associated with a low CBZ dose (hazard ratio 0.21, 95% CI 0.08-0.59, P = 0.002).
CONCLUSIONS: CBZ at a higher initial dose may have similar response rate and response duration, but longer survival duration after treatment with higher toxicity than a lower initial dose for docetaxel-resistant CRPC in Japanese patients.
METHODS: We retrospectively evaluated 118 patients who received CBZ for docetaxel-resistant CRPC in 10 university hospitals in Japan between 2014 and 2016. The rate of decrease of prostate-specific antigen (PSA), adverse events, progression-free survival (PFS), and overall survival (OS) were compared between patients receiving initially high (≥22.5 mg/m
RESULTS: PSA values decreased by > 50% in 22 patients (19%), with a higher frequency in the high-dose group than in the low-dose group (29 and 14%, P = 0.073). The median PFS time for the all-patient, high- and low-dose groups was 2.8 months (95% confidence interval [CI] 1.9-4.4), 2.1 months (1.2-5.5), and 3.0 months (2.0-4.4), respectively (P = 0.904). The median OS times were 16.3 months (95% CI 9.7-30.9), 30.9 months (11.8-47.4), and 10.2 months (8.6-20), respectively (P = 0.020). In multivariate analyses, PFS was significantly associated with existing bone metastasis at diagnosis (P = 0.005) and OS with PSA > 100 ng/ml (P = 0.007), hemoglobin < 12 g/dl (P = 0.030), and low initial CBZ dose (P = 0.030). Grade 4 neutropenia occurred in 53 patients (45%) and was associated with a low CBZ dose (hazard ratio 0.21, 95% CI 0.08-0.59, P = 0.002).
CONCLUSIONS: CBZ at a higher initial dose may have similar response rate and response duration, but longer survival duration after treatment with higher toxicity than a lower initial dose for docetaxel-resistant CRPC in Japanese patients.
Tucci M, Caffo O, Buttigliero C, et al.
Therapeutic options for first-line metastatic castration-resistant prostate cancer: Suggestions for clinical practise in the CHAARTED and LATITUDE era.
Cancer Treat Rev. 2019; 74:35-42 [PubMed] Related Publications
Therapeutic options for first-line metastatic castration-resistant prostate cancer: Suggestions for clinical practise in the CHAARTED and LATITUDE era.
Cancer Treat Rev. 2019; 74:35-42 [PubMed] Related Publications
In few years the scenario of metastatic prostate carcinoma treatment has radically changed due to improved knowledge of those mechanisms responsible of prostatic cancer cells survival and proliferation. Five new therapeutic agents (abiraterone acetate, enzalutamide, cabazitaxel, radium-223, sipuleucel-T), all able to improve overall survival, have been introduced in the management of metastatic castration-resistant prostate cancer. Moreover, recent evidences showed that adding docetaxel chemotherapy or abiraterone acetate to androgen deprivation therapy significantly increases overall survival of de novo castration-sensitive metastatic prostate cancer patients. Due to this rapid therapeutic evolution clinicians face one crucial challenge: the choice of the best treatment sequencing. In particular, there are no prospective data to guide clinical decision in patients with progressive disease after docetaxel or abiraterone acetate treatment for castration sensitive disease. In this review we provide an overview of the therapeutic agents available for both castration-sensitive and castration-resistant prostate cancer. We propose some biological and clinical insights helpful in selecting the most appropriate treatment for patients progressing after metastatic castration-sensitive prostate cancer treatment with docetaxel or abiraterone acetate.
Sun Y, Zhao Y, Teng S, et al.
Folic acid receptor-targeted human serum albumin nanoparticle formulation of cabazitaxel for tumor therapy.
Int J Nanomedicine. 2019; 14:135-148 [PubMed] Free Access to Full Article Related Publications
Folic acid receptor-targeted human serum albumin nanoparticle formulation of cabazitaxel for tumor therapy.
Int J Nanomedicine. 2019; 14:135-148 [PubMed] Free Access to Full Article Related Publications
Background: We previously developed cabazitaxel (CTX)-loaded human serum albumin nanoparticles (NPs-CTX) via a self-assembly method, and these NPs showed efficacy in prostate cancer therapy. Many studies have shown that the levels of folic acid (FA) receptor on the surface of various tumor cells are high. Therefore, FA-modified NPs-CTX may have enhanced antitumor effects compared with unmodified NPs-CTX.
Methods: NPs-CTX were first prepared via self-assembly, and FA was conjugated on the surface of NPs-CTX through the -NH
Results: Both NPs-CTX and FA-NPs-CTX exhibited good stability and morphology. Drug release from the NPs was not affected by FA conjugation. Compared with CTX dissolved in a mixture of Tween 80 and 13% ethanol (w/w) at a ratio of 1:4 (v/v) (Tween-CTX), the two nanoformulations had lower lytic activity against normal red blood cells. However, FA-NPs-CTX showed greater inhibition of tumor cells with overexpressed FR, compared with NPs-CTX, in the cytotoxicity experiments. Moreover, the cellular uptake of FA-NPs-CTX was enhanced through FR-mediated endocytosis in HeLa cells in vitro and HeLa xenograft tumors in vivo. Although Tween-CTX exhibited tumor growth inhibition similar to FA-NPs-CTX in vivo, this inhibition also caused adverse side effects; the median lethal dose (LD50) of Tween-CTX to mice was 5.68 mg/kg, while FA-NPs-CTX-treated mice survived at doses exceeding 400 mg/kg.
Conclusion: The results showed that FA-NPs-CTX caused inhibition of tumor growth in a manner similar to that of Tween-CTX; however, the safety and tolerability of CTX were greatly improved by FA conjugation compared with those of Tween-CTX. In summary, FA-NPs-CTX have great potential in CTX delivery, and this formulation is a promising candidate for the treatment of cancers with high FR levels.
Methods: NPs-CTX were first prepared via self-assembly, and FA was conjugated on the surface of NPs-CTX through the -NH
Results: Both NPs-CTX and FA-NPs-CTX exhibited good stability and morphology. Drug release from the NPs was not affected by FA conjugation. Compared with CTX dissolved in a mixture of Tween 80 and 13% ethanol (w/w) at a ratio of 1:4 (v/v) (Tween-CTX), the two nanoformulations had lower lytic activity against normal red blood cells. However, FA-NPs-CTX showed greater inhibition of tumor cells with overexpressed FR, compared with NPs-CTX, in the cytotoxicity experiments. Moreover, the cellular uptake of FA-NPs-CTX was enhanced through FR-mediated endocytosis in HeLa cells in vitro and HeLa xenograft tumors in vivo. Although Tween-CTX exhibited tumor growth inhibition similar to FA-NPs-CTX in vivo, this inhibition also caused adverse side effects; the median lethal dose (LD50) of Tween-CTX to mice was 5.68 mg/kg, while FA-NPs-CTX-treated mice survived at doses exceeding 400 mg/kg.
Conclusion: The results showed that FA-NPs-CTX caused inhibition of tumor growth in a manner similar to that of Tween-CTX; however, the safety and tolerability of CTX were greatly improved by FA conjugation compared with those of Tween-CTX. In summary, FA-NPs-CTX have great potential in CTX delivery, and this formulation is a promising candidate for the treatment of cancers with high FR levels.
Mahira S, Kommineni N, Husain GM, Khan W
Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: A new insight into nanomedicine based combinational chemotherapy for prostate cancer.
Biomed Pharmacother. 2019; 110:803-817 [PubMed] Related Publications
Cabazitaxel and silibinin co-encapsulated cationic liposomes for CD44 targeted delivery: A new insight into nanomedicine based combinational chemotherapy for prostate cancer.
Biomed Pharmacother. 2019; 110:803-817 [PubMed] Related Publications
Cancer stem cells (CSCs) are the promising targets for cancer chemotherapy that cannot be eliminated by conventional chemotherapy. In this study cationic liposomes of cabazitaxel (CBX) and silibinin (SIL) were prepared with an aim to kill cancer cells and CSCs for prostate cancer. CBX act as cancer cell inhibitor and SIL as CSC inhibitor. Hyaluronic acid (HA), an endogenous anionic polysaccharide was coated on cationic liposomes for targeting CD44 receptors over expressed on CSCs. Liposomes were prepared by ethanol injection method with particle size below 100 nm and entrapment efficiency of more than 90% at 10% w/w drug loading. Liposomes were characterized by dynamic light scattering, transmission electron microscopy,
El Bairi K, Atanasov AG, Amrani M, Afqir S
The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery.
Biomed Pharmacother. 2019; 109:2492-2498 [PubMed] Related Publications
The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery.
Biomed Pharmacother. 2019; 109:2492-2498 [PubMed] Related Publications
Intrinsic or acquired drug resistance, adverse drug reactions and tumor heterogeneity between and within cancer patients limit the efficacy of clinical management of advanced cancers. To overcome these barriers, predictive biomarkers have recently emerged to guide medical oncologists in the selection of cancer patients who will respond to various anticancer treatments and to improve the toxicity to benefit ratio. Notably, targeted therapy has significantly benefited from these advances, but the application of predictive biomarkers have been a bit slower with some drugs derived from natural sources such as trabectedin, cabazitaxel and alvocidib. In this paper, we discuss some recent advances regarding the use of cancer biomarkers to predict efficacy of some selected natural compounds with a focus on human clinical studies.
Beaumont H, Evans TL, Klifa C, et al.
Discrepancies of assessments in a RECIST 1.1 phase II clinical trial - association between adjudication rate and variability in images and tumors selection.
Cancer Imaging. 2018; 18(1):50 [PubMed] Free Access to Full Article Related Publications
Discrepancies of assessments in a RECIST 1.1 phase II clinical trial - association between adjudication rate and variability in images and tumors selection.
Cancer Imaging. 2018; 18(1):50 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: In imaging-based clinical trials, it is common practice to perform double reads for each image, discrepant interpretations can result from these two different evaluations. In this study we analyzed discrepancies that occurred between local investigators (LI) and blinded independent central review (BICR) by comparing reader-selected imaging scans and lesions. Our goal was to identify the causes of discrepant declarations of progressive disease (PD) between LI and BICR in a clinical trial.
METHODS: We retrospectively analyzed imaging data from a RECIST 1.1-based, multi-sites, phase II clinical trial of 179 patients with adult small cell lung cancer, treated with Cabazitaxel compared to Topotecan. Any discrepancies in the determination of PD between LI and BICR readers were reviewed by a third-party adjudicator. For each imaging time point and reader, we recorded the selected target lesions, non-target lesions, and new lesions. Odds ratios were calculated to measure the association between discrepant declarations of PD and the differences in reviewed imaging scans (e.g. same imaging modality but with different reconstruction parameters) and selected lesions. Reasons for discrepancies were analyzed.
RESULTS: The average number of target lesions found by LI and BICR was respectively 2.9 and 3.4 per patient (p < 0.05), 18.4% of these target lesions were actually non-measurable. LI and BICR performed their evaluations based on different baseline imaging scans for 59% of the patients, they selected at least one different target lesion in 85% of patients. A total of 36.7% of patients required adjudication. Reasons of adjudication included differences in 1) reporting new lesions (53.7%), 2) the measured change of the tumor burden (18.5%), and 3) the progression of non-target lesions (11.2%). The rate of discrepancy was not associated with the selection of non-measurable target lesions or with the readers' assessment of different images. Paradoxically, more discrepancies occurred when LI and BICR selected exactly the same target lesions at baseline compared to when readers selected not exactly the same lesions.
CONCLUSIONS: For a large proportion of evaluations, LI and BICR did not select the same imaging scans and target lesions but with a limited impact on the rate of discrepancy. The majority of discrepancies were explained by the difference in detecting new lesions.
TRIAL REGISTRATION: ARD12166 ( https://clinicaltrials.gov/ct2/show/NCT01500720 ).
METHODS: We retrospectively analyzed imaging data from a RECIST 1.1-based, multi-sites, phase II clinical trial of 179 patients with adult small cell lung cancer, treated with Cabazitaxel compared to Topotecan. Any discrepancies in the determination of PD between LI and BICR readers were reviewed by a third-party adjudicator. For each imaging time point and reader, we recorded the selected target lesions, non-target lesions, and new lesions. Odds ratios were calculated to measure the association between discrepant declarations of PD and the differences in reviewed imaging scans (e.g. same imaging modality but with different reconstruction parameters) and selected lesions. Reasons for discrepancies were analyzed.
RESULTS: The average number of target lesions found by LI and BICR was respectively 2.9 and 3.4 per patient (p < 0.05), 18.4% of these target lesions were actually non-measurable. LI and BICR performed their evaluations based on different baseline imaging scans for 59% of the patients, they selected at least one different target lesion in 85% of patients. A total of 36.7% of patients required adjudication. Reasons of adjudication included differences in 1) reporting new lesions (53.7%), 2) the measured change of the tumor burden (18.5%), and 3) the progression of non-target lesions (11.2%). The rate of discrepancy was not associated with the selection of non-measurable target lesions or with the readers' assessment of different images. Paradoxically, more discrepancies occurred when LI and BICR selected exactly the same target lesions at baseline compared to when readers selected not exactly the same lesions.
CONCLUSIONS: For a large proportion of evaluations, LI and BICR did not select the same imaging scans and target lesions but with a limited impact on the rate of discrepancy. The majority of discrepancies were explained by the difference in detecting new lesions.
TRIAL REGISTRATION: ARD12166 ( https://clinicaltrials.gov/ct2/show/NCT01500720 ).
Han X, Gong F, Chi L, et al.
Cancer-targeted and glutathione-responsive micellar carriers for controlled delivery of cabazitaxel.
Nanotechnology. 2019; 30(5):055601 [PubMed] Related Publications
Cancer-targeted and glutathione-responsive micellar carriers for controlled delivery of cabazitaxel.
Nanotechnology. 2019; 30(5):055601 [PubMed] Related Publications
Novel type of multifunctional polymeric micelles (PMs) designated as HM-PMss/CTX micelles were developed in the present study for tumor-targeted and glutathione (GSH)-responsive delivery of cabazitaxel (CTX). The surface of the vehicles was modified with piloting molecules (HM-3 peptide), which targets α
Cheng Y, Ou Z, Li Q, et al.
Cabazitaxel liposomes with aptamer modification enhance tumor‑targeting efficacy in nude mice.
Mol Med Rep. 2019; 19(1):490-498 [PubMed] Free Access to Full Article Related Publications
Cabazitaxel liposomes with aptamer modification enhance tumor‑targeting efficacy in nude mice.
Mol Med Rep. 2019; 19(1):490-498 [PubMed] Free Access to Full Article Related Publications
The present study investigated the feasibility of improving the tumor‑targeting efficacy and decreasing the toxicity of liposomal cabazitaxel (Cab) with aptamer modification. The process involved preparing aptamer (TLS1c)‑modified liposomes and studying the behavior of the liposomes in vitro and in vivo. TLS1c as an aptamer, which has high specificity for BNL 1ME A.7R.1 (MEAR) cells, was conjugated with Cab liposomes (Cab/lipo) to enhance MEAR tumor tissue targeting. Confocal laser scanning microscopy and flow cytometry analyses demonstrated that the fluorescence of the liposomes modified with the aptamer was notably stronger compared with that of the unmodified liposomes. Furthermore, the biodistribution data of the modified liposomes tested in tumor‑bearing mice revealed high specificity of biotinylated TLS1c‑modified Cab/lipo (BioTL‑Cab/lipo) for tumor tissues. Furthermore, the modified liposomes demonstrated decreased cytotoxicity and simultaneously retained potent inhibition against tumor growth. It is likely that the specific binding of the aptamer (TLS1c) to the targeted cells (MEAR) facilitates the binding of the liposomes to the targeted cells. Therefore, BioTL‑Cab/lipo may be considered as a promising delivery system to improve cell targeting and reduce drug toxicity in the treatment of cancer.
Ingrosso G, Detti B, Scartoni D, et al.
Current therapeutic options in metastatic castration-resistant prostate cancer.
Semin Oncol. 2018; 45(5-6):303-315 [PubMed] Related Publications
Current therapeutic options in metastatic castration-resistant prostate cancer.
Semin Oncol. 2018; 45(5-6):303-315 [PubMed] Related Publications
BACKGROUND: The tumors of many patients with prostate cancer eventually become refractory to androgen deprivation therapy with progression to metastatic castration-resistant disease. Significant advances in the treatment of metastatic castration-resistant prostate cancer (mCRPC) have been made in recent years, and new treatment strategies have recently been made available. The aim of this report was to schematically review all the approved pharmacologic treatment options for patients with mCRPC through 2018, analyzing the efficacy and possible side effects of each therapy to assist clinicians in reaching an appropriate treatment decision. New biomarkers potentially of aid in the choice of treatment in this setting are also briefly reviewed.
METHODS: We performed a literature search of clinical trials of new drugs and treatments for patients diagnosed with mCRPC published through 2018.
RESULTS: Two new hormonal drugs, abiraterone acetate and enzalutamide have been approved by FDA in 2011 and 2012, respectively for the treatment of patients with mCRPC and have undergone extensive testing. While these treatments have shown a benefit in progression-free and overall survival, the appropriate sequencing must still be determined so that treatment decisions can be made based on their specific clinical profile. Cabazitaxel has been shown to be an efficient therapeutic option in a postdocetaxel setting, while its role in chemotherapy-naïve patients must still be determined. Sipuleucel-T and radium-223 have been studied in patients without visceral metastases and have achieved overall survival benefits with good safety profiles. The feasibility and efficacy of combinations of new treatments with other known therapies such as chemotherapy are currently under investigation.
CONCLUSIONS: Drug development efforts continue to attempt to prolong survival and improve quality of life in the mCRPC setting, with several therapeutic options available. Ongoing and future trials are needed to further assess the efficacy and safety of these new drugs and their interactions, along with the most appropriate sequencing.
METHODS: We performed a literature search of clinical trials of new drugs and treatments for patients diagnosed with mCRPC published through 2018.
RESULTS: Two new hormonal drugs, abiraterone acetate and enzalutamide have been approved by FDA in 2011 and 2012, respectively for the treatment of patients with mCRPC and have undergone extensive testing. While these treatments have shown a benefit in progression-free and overall survival, the appropriate sequencing must still be determined so that treatment decisions can be made based on their specific clinical profile. Cabazitaxel has been shown to be an efficient therapeutic option in a postdocetaxel setting, while its role in chemotherapy-naïve patients must still be determined. Sipuleucel-T and radium-223 have been studied in patients without visceral metastases and have achieved overall survival benefits with good safety profiles. The feasibility and efficacy of combinations of new treatments with other known therapies such as chemotherapy are currently under investigation.
CONCLUSIONS: Drug development efforts continue to attempt to prolong survival and improve quality of life in the mCRPC setting, with several therapeutic options available. Ongoing and future trials are needed to further assess the efficacy and safety of these new drugs and their interactions, along with the most appropriate sequencing.
Natsagdorj A, Izumi K, Hiratsuka K, et al.
CCL2 induces resistance to the antiproliferative effect of cabazitaxel in prostate cancer cells.
Cancer Sci. 2019; 110(1):279-288 [PubMed] Free Access to Full Article Related Publications
CCL2 induces resistance to the antiproliferative effect of cabazitaxel in prostate cancer cells.
Cancer Sci. 2019; 110(1):279-288 [PubMed] Free Access to Full Article Related Publications
Understanding the mechanism of chemoresistance and disease progression in patients with prostate cancer is important for developing novel treatment strategies. In particular, developing resistance to cabazitaxel is a major challenge in patients with docetaxel-resistant and castration-resistant prostate cancer (CRPC) because cabazitaxel is often administered as a last resort. However, the mechanism by which cabazitaxel resistance develops is still unclear. C-C motif chemokine ligands (CCL) were shown to contribute to the castration resistance of prostate cancer cells via an autocrine mechanism. Therefore, we focused on CCL as key factors of chemoresistance in prostate cancer cells. We previously established a cabazitaxel-resistant cell line, DU145-TxR/CxR, from a previously established paclitaxel-resistant cell line, DU145-TxR. cDNA microarray analysis revealed that the expression of CCL2 was upregulated in both DU145-TxR and DU145-TxR/CxR cells compared with DU145 cells. The secreted CCL2 protein level in DU145-TxR and DU145-TxR/CxR cells was also higher than in parental DU145 cells. The stimulation of DU145 cells with CCL2 increased the proliferation rate under treatments with cabazitaxel, and a CCR2 (a specific receptor of CCL2) antagonist suppressed the proliferation of DU145-TxR and DU145-TxR/CxR cells under treatments of cabazitaxel. The CCL2-CCR2 axis decreased apoptosis through the inhibition of caspase-3 and poly(ADP-ribose) polymerase (PARP). CCL2 is apparently a key contributor to cabazitaxel resistance in prostate cancer cells. Inhibition of the CCL2-CCR2 axis may be a potential therapeutic strategy against chemoresistant CRPC in combination with cabazitaxel.
Batra A, Winquist E
Emerging cell cycle inhibitors for treating metastatic castration-resistant prostate cancer.
Expert Opin Emerg Drugs. 2018; 23(4):271-282 [PubMed] Related Publications
Emerging cell cycle inhibitors for treating metastatic castration-resistant prostate cancer.
Expert Opin Emerg Drugs. 2018; 23(4):271-282 [PubMed] Related Publications
INTRODUCTION: Disease progression despite androgen suppression defines lethal castration-resistant prostate cancer (CRPC). Most of these cancers remain androgen receptor (AR)-signaling dependent. Therapy for metastatic CRPC includes abiraterone acetate, enzalutamide, docetaxel, cabazitaxel, sipuleucel-T, and radium-223. However, survival remains modest for men with progressive disease despite AR-targeted therapy and docetaxel, and therefore novel treatments are needed. Areas covered: Recent evidence of genomic heterogeneity and sensitivity to PARP inhibitors supports investigation of targeted agents in CRPC. Cell cycle inhibitors are therefore logical molecules to investigate. Review of the current literature identified cell cycle inhibitors under study in early phase clinical trials targeting the G1 (palbociclib, ribociclib, AZD-5363, ipatasertib), S (M-6620, prexasertib), G2 (adavosertib), and M (alisertib) phases of the cell cycle. Expert opinion: Strategies combining cell cycle inhibitors with active agents in CRPC are most likely to have clinical impact with CDK4/6 and Wee1 inhibitors appearing most promising. Identification of predictive biomarkers may be essential and currently trials are testing circulating cell-free DNA as an approach. Incremental toxicities such as neutropenia are important in this population. Results from most current clinical trials of cell cycle inhibitors in CRPC are still pending but it is anticipated they will provide important insights into the heterogeneous biology of CRPC.
Sak K, Lust H, Kase M, et al.
Suppression of Taxanes Cytotoxicity by Citrus Flavonoid Hesperetin in PPC-1 Human Prostate Cancer Cells.
Anticancer Res. 2018; 38(11):6209-6215 [PubMed] Related Publications
Suppression of Taxanes Cytotoxicity by Citrus Flavonoid Hesperetin in PPC-1 Human Prostate Cancer Cells.
Anticancer Res. 2018; 38(11):6209-6215 [PubMed] Related Publications
BACKGROUND/AIM: More than half of prostate cancer patients use, in addition to conventional therapies, some kind of complementary medicine, including flavonoid-rich products. However, knowledge about the co-effects of flavonoids with cytotoxic chemotherapies is still rather poor. Therefore, this study was undertaken to assess the cytotoxic activity of flavonoids and their interactions with taxanes in human advanced prostate cancer cells.
MATERIALS AND METHODS: Cytotoxicity of different flavonoids and their effects on the efficacy of docetaxel and cabazitaxel were studied in the human metastatic prostate cancer cell line PPC-1, using MTT colorimetric assay.
RESULTS: Both taxanes suppressed the viability of PPC-1 cells with IC
CONCLUSION: Flavonoid hesperetin remarkably suppressed the cytotoxic efficacy of taxanes in prostate cancer cells. Therefore, caution is required from prostate cancer patients who take hesperetin-containing oral supplements.
MATERIALS AND METHODS: Cytotoxicity of different flavonoids and their effects on the efficacy of docetaxel and cabazitaxel were studied in the human metastatic prostate cancer cell line PPC-1, using MTT colorimetric assay.
RESULTS: Both taxanes suppressed the viability of PPC-1 cells with IC
CONCLUSION: Flavonoid hesperetin remarkably suppressed the cytotoxic efficacy of taxanes in prostate cancer cells. Therefore, caution is required from prostate cancer patients who take hesperetin-containing oral supplements.
Su F, Ahn S, Saha A, et al.
Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance.
Oncogene. 2019; 38(11):1979-1988 [PubMed] Free Access to Full Article Related Publications
Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance.
Oncogene. 2019; 38(11):1979-1988 [PubMed] Free Access to Full Article Related Publications
Fat tissue, overgrowing in obesity, promotes the progression of various carcinomas. Clinical and animal model studies indicate that adipose stromal cells (ASC), the progenitors of adipocytes, are recruited by tumors and promote tumor growth as tumor stromal cells. Here, we investigated the role of ASC in cancer chemoresistance and invasiveness, the attributes of tumor aggressiveness. By using human cell co-culture models, we demonstrate that ASC induce epithelial-mesenchymal transition (EMT) in prostate cancer cells. Our results for the first time demonstrate that ASC interaction renders cancer cells more migratory and resistant to docetaxel, cabazitaxel, and cisplatin chemotherapy. To confirm these findings in vivo, we compared cancer aggressiveness in lean and obese mice grafted with prostate tumors. We show that obesity promotes EMT in cancer cells and tumor invasion into the surrounding fat tissue. A hunter-killer peptide D-CAN, previously developed for targeted ASC ablation, suppressed the obesity-associated EMT and cancer progression. Importantly, cisplatin combined with D-CAN was more effective than cisplatin alone in suppressing growth of mouse prostate cancer allografts and xenografts even in non-obese mice. Our data demonstrate that ASC promote tumor aggressiveness and identify them as a target of combination cancer therapy.
Sathianathen NJ, Philippou YA, Kuntz GM, et al.
Taxane-based chemohormonal therapy for metastatic hormone-sensitive prostate cancer.
Cochrane Database Syst Rev. 2018; 10:CD012816 [PubMed] Article available free on PMC after 15/10/2019 Related Publications
Taxane-based chemohormonal therapy for metastatic hormone-sensitive prostate cancer.
Cochrane Database Syst Rev. 2018; 10:CD012816 [PubMed] Article available free on PMC after 15/10/2019 Related Publications
BACKGROUND: There has been considerable development in the treatment of advanced prostate cancer over the last decade. A number of agents, including docetaxel, cabazitaxel, abiraterone acetate, enzalutamide and sipuleucel-T, have been reported to improve outcomes in men with castration-resistant disease and their use is being explored in hormone-sensitive prostate cancer.
OBJECTIVES: To assess the effects of early taxane-based chemohormonal therapy for newly diagnosed, metastatic, hormone-sensitive prostate cancer.
SEARCH METHODS: We performed a comprehensive search using multiple databases (the Cochrane Library, MEDLINE, Embase, Scopus, Google Scholar, and Web of Science), trials registries, other sources of grey literature, and conference proceedings, up to 10 August 2018. We applied no restrictions on publication language or status.
SELECTION CRITERIA: We included randomized or quasi-randomized controlled trials in which participants were administered taxane-based chemotherapy with systemic androgen deprivation therapy (ADT) within 120 days of beginning ADT versus ADT alone at the time of diagnosis of metastatic disease.
DATA COLLECTION AND ANALYSIS: Two review authors independently classified studies and abstracted data from the included studies. We performed statistical analyses using a random-effects model. We rated the quality of evidence according to the GRADE approach.
MAIN RESULTS: The search identified three studies in which 2,261 participants were randomized to receive either ADT alone, or taxane-based chemotherapy at a dose of 75mg per square meter of body surface area at three-weekly intervals for six or nine cycles in addition to ADT.Primary outcomesEarly treatment with taxane-based chemotherapy in addition to ADT probably reduces death from any cause compared to ADT alone (hazard ratio (HR) 0.77, 95% confidence interval (CI) 0.68 to 0.87; moderate-certainty evidence); this would result in 94 fewer deaths per 1,000 men (95% CI 51 to 137 fewer deaths). We downgraded the certainty of evidence due to study limitations related to potential performance bias. Based on the results of one study with 375 participants, the addition of taxane-based chemotherapy to ADT may increase the incidence of Grade III to V adverse events compared to ADT alone (risk ratio (RR) 2.98, 95% CI 2.19 to 4.04; low-certainty evidence); this would result in 405 more Grade III to V adverse events per 1,000 men (95% CI 243 to 621 more events). We downgraded the certainty of evidence due to study limitations and imprecision.Secondary outcomesEarly taxane-based chemotherapy in addition to ADT probably reduces the risk of prostate cancer-specific death (RR 0.79, 95% CI 0.70 to 0.89; moderate-certainty evidence). We downgraded the certainty of evidence due to study limitations related to potential performance and detection bias. The addition of taxane-based chemotherapy also probably reduces disease progression compared to ADT alone (HR 0.63, 95% CI 0.56 to 0.71; moderate-certainty evidence). We downgraded the certainty of evidence because of study limitations related to potential performance bias. The addition of taxane-based chemotherapy to ADT may result in a large increase in the risk of treatment discontinuation due to adverse events (RR 79.41, 95% CI 4.92 to 1282.78; low-certainty evidence). We downgraded the certainty of evidence due to study limitations and imprecision. This estimate is derived from a single study with no events in the control arm but a discontinuation rate of 20% in the intervention arm. Taxane-based chemotherapy may increase the incidence of adverse events of any grade (RR 1.11, 95% CI 1.06 to 1.17; low-certainty evidence). We downgraded our assessment of the certainty of evidence due to very serious study limitations. There may be a small improvement, which may not be clinically important, in quality of life at 12 months with combination treatment (mean difference (MD) 2.85 on the Functional Assessment of Cancer Therapy-Prostate scale, 95% CI 0.13 higher to 5.57 higher; low-certainty evidence). We downgraded the certainty of evidence for study limitations related to potential performance, detection and attrition bias.
AUTHORS' CONCLUSIONS: Compared to ADT alone, the early (within 120 days of beginning ADT) addition of taxane-based chemotherapy to ADT for hormone-sensitive prostate cancer probably prolongs both overall and disease-specific survival and delays disease progression. There may be an increase in toxicity with taxane-based chemotherapy in combination with ADT. There may also be a small, clinically unimportant improvement in quality of life at 12 months with taxane-based chemotherapy and ADT treatment.
OBJECTIVES: To assess the effects of early taxane-based chemohormonal therapy for newly diagnosed, metastatic, hormone-sensitive prostate cancer.
SEARCH METHODS: We performed a comprehensive search using multiple databases (the Cochrane Library, MEDLINE, Embase, Scopus, Google Scholar, and Web of Science), trials registries, other sources of grey literature, and conference proceedings, up to 10 August 2018. We applied no restrictions on publication language or status.
SELECTION CRITERIA: We included randomized or quasi-randomized controlled trials in which participants were administered taxane-based chemotherapy with systemic androgen deprivation therapy (ADT) within 120 days of beginning ADT versus ADT alone at the time of diagnosis of metastatic disease.
DATA COLLECTION AND ANALYSIS: Two review authors independently classified studies and abstracted data from the included studies. We performed statistical analyses using a random-effects model. We rated the quality of evidence according to the GRADE approach.
MAIN RESULTS: The search identified three studies in which 2,261 participants were randomized to receive either ADT alone, or taxane-based chemotherapy at a dose of 75mg per square meter of body surface area at three-weekly intervals for six or nine cycles in addition to ADT.Primary outcomesEarly treatment with taxane-based chemotherapy in addition to ADT probably reduces death from any cause compared to ADT alone (hazard ratio (HR) 0.77, 95% confidence interval (CI) 0.68 to 0.87; moderate-certainty evidence); this would result in 94 fewer deaths per 1,000 men (95% CI 51 to 137 fewer deaths). We downgraded the certainty of evidence due to study limitations related to potential performance bias. Based on the results of one study with 375 participants, the addition of taxane-based chemotherapy to ADT may increase the incidence of Grade III to V adverse events compared to ADT alone (risk ratio (RR) 2.98, 95% CI 2.19 to 4.04; low-certainty evidence); this would result in 405 more Grade III to V adverse events per 1,000 men (95% CI 243 to 621 more events). We downgraded the certainty of evidence due to study limitations and imprecision.Secondary outcomesEarly taxane-based chemotherapy in addition to ADT probably reduces the risk of prostate cancer-specific death (RR 0.79, 95% CI 0.70 to 0.89; moderate-certainty evidence). We downgraded the certainty of evidence due to study limitations related to potential performance and detection bias. The addition of taxane-based chemotherapy also probably reduces disease progression compared to ADT alone (HR 0.63, 95% CI 0.56 to 0.71; moderate-certainty evidence). We downgraded the certainty of evidence because of study limitations related to potential performance bias. The addition of taxane-based chemotherapy to ADT may result in a large increase in the risk of treatment discontinuation due to adverse events (RR 79.41, 95% CI 4.92 to 1282.78; low-certainty evidence). We downgraded the certainty of evidence due to study limitations and imprecision. This estimate is derived from a single study with no events in the control arm but a discontinuation rate of 20% in the intervention arm. Taxane-based chemotherapy may increase the incidence of adverse events of any grade (RR 1.11, 95% CI 1.06 to 1.17; low-certainty evidence). We downgraded our assessment of the certainty of evidence due to very serious study limitations. There may be a small improvement, which may not be clinically important, in quality of life at 12 months with combination treatment (mean difference (MD) 2.85 on the Functional Assessment of Cancer Therapy-Prostate scale, 95% CI 0.13 higher to 5.57 higher; low-certainty evidence). We downgraded the certainty of evidence for study limitations related to potential performance, detection and attrition bias.
AUTHORS' CONCLUSIONS: Compared to ADT alone, the early (within 120 days of beginning ADT) addition of taxane-based chemotherapy to ADT for hormone-sensitive prostate cancer probably prolongs both overall and disease-specific survival and delays disease progression. There may be an increase in toxicity with taxane-based chemotherapy in combination with ADT. There may also be a small, clinically unimportant improvement in quality of life at 12 months with taxane-based chemotherapy and ADT treatment.
von Hardenberg J, Worst TS, Westhoff N, et al.
Cell-Free DNA and Neuromediators in Detecting Aggressive Variant Prostate Cancer.
Oncol Res Treat. 2018; 41(10):627-633 [PubMed] Related Publications
Cell-Free DNA and Neuromediators in Detecting Aggressive Variant Prostate Cancer.
Oncol Res Treat. 2018; 41(10):627-633 [PubMed] Related Publications
BACKGROUND: Aggressive variant transformation in metastatic castration-resistant prostate cancer (mCRPC) represents an under-recognized phenomenon. There is an urgent need for non-invasive biomarkers to detect these variants and identify treatment alternatives.
METHODS: A prospective observational pilot study in mCRPC patients receiving treatment with cabazitaxel (CAB) was conducted. Neuromediators were sequentially evaluated and their impact on disease endpoints calculated. Targeted next-generation sequencing (NGS) of cell-free DNA (cfDNA) was also performed in a highly pretreated subset of patients.
RESULTS: 23 patients were included. Estimated effects indicate that neuron-specific enolase (NSE) levels at baseline may be correlated with overall survival (NSE unit 18.3 ng/ml: HR1.262 (95% confidence interval (CI) 0.985-1.616)) and that chromogranin A (CGA) may be correlated with progression-free survival (CGA unit 98.1 ng/ml: HR1.341 (95% CI 1.011-1.778)). cfDNA analysis revealed mutations annotated in prostate cancer (PCA) and small cell cancers (SCC). 1 patient showed elevated neuromediators along with annotated mutations in PCA and SCC, potentially indicating aggressive variant cancer. In 3 patients KIT mutations (e.g. pM541L, pV654A) known to be tissue-based biomarkers with level 1 evidence for the treatment with imatinib and sunitinib were found.
CONCLUSIONS: Sequential analysis of neuromediators and targeted NGS of cfDNA provide insight for the estimation of tumor heterogeneity under therapy with CAB.
METHODS: A prospective observational pilot study in mCRPC patients receiving treatment with cabazitaxel (CAB) was conducted. Neuromediators were sequentially evaluated and their impact on disease endpoints calculated. Targeted next-generation sequencing (NGS) of cell-free DNA (cfDNA) was also performed in a highly pretreated subset of patients.
RESULTS: 23 patients were included. Estimated effects indicate that neuron-specific enolase (NSE) levels at baseline may be correlated with overall survival (NSE unit 18.3 ng/ml: HR1.262 (95% confidence interval (CI) 0.985-1.616)) and that chromogranin A (CGA) may be correlated with progression-free survival (CGA unit 98.1 ng/ml: HR1.341 (95% CI 1.011-1.778)). cfDNA analysis revealed mutations annotated in prostate cancer (PCA) and small cell cancers (SCC). 1 patient showed elevated neuromediators along with annotated mutations in PCA and SCC, potentially indicating aggressive variant cancer. In 3 patients KIT mutations (e.g. pM541L, pV654A) known to be tissue-based biomarkers with level 1 evidence for the treatment with imatinib and sunitinib were found.
CONCLUSIONS: Sequential analysis of neuromediators and targeted NGS of cfDNA provide insight for the estimation of tumor heterogeneity under therapy with CAB.
Saka C
Chromatographic Methods for Determination of Drugs Used in Prostate Cancer in Biological and Pharmacological Samples.
Crit Rev Anal Chem. 2019; 49(1):78-99 [PubMed] Related Publications
Chromatographic Methods for Determination of Drugs Used in Prostate Cancer in Biological and Pharmacological Samples.
Crit Rev Anal Chem. 2019; 49(1):78-99 [PubMed] Related Publications
Prostate cancer is the second most common cancer and the fifth leading cause of cancer deaths in men worldwide. This review article contains a summary of analyzes performed by chromatographic methods used for the determination of abiraterone acetate, bicalutamide, cabazitaxel, docetaxel, enzalutamide, flutamide, goserelin acetate, leuprolide acetate, and mitoxantrone hydrochloride drugs used in prostate cancer applications in biological and pharmacological samples. In this review, sample preparation procedures, chromatographic procedures, and detectors used for analytical determinations of these drugs are discussed.
Kosaka T, Hongo H, Watanabe K, et al.
No significant impact of patient age and prior treatment profile with docetaxel on the efficacy of cabazitaxel in patient with castration-resistant prostate cancer.
Cancer Chemother Pharmacol. 2018; 82(6):1061-1066 [PubMed] Article available free on PMC after 15/10/2019 Related Publications
No significant impact of patient age and prior treatment profile with docetaxel on the efficacy of cabazitaxel in patient with castration-resistant prostate cancer.
Cancer Chemother Pharmacol. 2018; 82(6):1061-1066 [PubMed] Article available free on PMC after 15/10/2019 Related Publications
BACKGROUND: The correlation of the oncological outcomes of docetaxel and cabazitaxel in Japanese metastatic castration-resistant prostate cancer (mCRPC) patients has not been unclear.
MATERIALS AND METHODS: This study included a total of 47 consecutive Japanese mCRPC patients treated with cabazitaxel and assessed the prognostic significance of cabazitaxel, focusing on patient age and the correlation of efficacy between docetaxel and cabazitaxel.
RESULTS: Prostate-specific antigen (PSA) decline was observed in 27 patients (57.4%), including 19 (40.0%) achieving the response defined by PSA decline ≥ 30%. The median overall survival (OS) periods after the introduction of cabazitaxel was 16.1 months. Twenty (42.6%) were judged to have responded to cabazitaxel with a PSA decrease ≥ 30% from the baseline. A 30% PSA response to cabazitaxel was achieved in 4 (50.0%) patients with ≧ 75 years (n = 8) and 16 (41.0%) patients with less than 75 years (n = 39). There was no significant correlation between the PSA response and patients' age (p = 0.707). A 30% PSA response to cabazitaxel was achieved in 13 (46.4%) and 7 (36.8%) patients with and without that to docetaxel, respectively. A 30% PSA response to cabazitaxel was achieved in 5 (16.6%) and 7 (41.2%) patients who had treated with less than 10 cycles docetaxel or 10 ≦ cycles, respectively. Univariate and multivariate analyses revealed that there were no significant correlation of patient age (p = 0.537), the response to prior docetaxel therapy (p = 0.339) or cycles of docetaxel therapy (p = 0.379) with shorter OS.
CONCLUSION: These results indicate that the introduction of cabazitaxel for Japanese mCRPC patients could result in oncological outcomes without any association with patient's age and the profiles of previous docetaxel therapy.
MATERIALS AND METHODS: This study included a total of 47 consecutive Japanese mCRPC patients treated with cabazitaxel and assessed the prognostic significance of cabazitaxel, focusing on patient age and the correlation of efficacy between docetaxel and cabazitaxel.
RESULTS: Prostate-specific antigen (PSA) decline was observed in 27 patients (57.4%), including 19 (40.0%) achieving the response defined by PSA decline ≥ 30%. The median overall survival (OS) periods after the introduction of cabazitaxel was 16.1 months. Twenty (42.6%) were judged to have responded to cabazitaxel with a PSA decrease ≥ 30% from the baseline. A 30% PSA response to cabazitaxel was achieved in 4 (50.0%) patients with ≧ 75 years (n = 8) and 16 (41.0%) patients with less than 75 years (n = 39). There was no significant correlation between the PSA response and patients' age (p = 0.707). A 30% PSA response to cabazitaxel was achieved in 13 (46.4%) and 7 (36.8%) patients with and without that to docetaxel, respectively. A 30% PSA response to cabazitaxel was achieved in 5 (16.6%) and 7 (41.2%) patients who had treated with less than 10 cycles docetaxel or 10 ≦ cycles, respectively. Univariate and multivariate analyses revealed that there were no significant correlation of patient age (p = 0.537), the response to prior docetaxel therapy (p = 0.339) or cycles of docetaxel therapy (p = 0.379) with shorter OS.
CONCLUSION: These results indicate that the introduction of cabazitaxel for Japanese mCRPC patients could result in oncological outcomes without any association with patient's age and the profiles of previous docetaxel therapy.
Maines F, De Giorgi U, Procopio G, et al.
Enzalutamide after chemotherapy in advanced castration-resistant prostate cancer: the Italian Named Patient Program.
Future Oncol. 2018; 14(26):2691-2699 [PubMed] Related Publications
Enzalutamide after chemotherapy in advanced castration-resistant prostate cancer: the Italian Named Patient Program.
Future Oncol. 2018; 14(26):2691-2699 [PubMed] Related Publications
AIM: To collect efficacy and safety data of enzalutamide after docetaxel, we retrospectively evaluated the Italian Named Patient Program results.
PATIENTS & METHODS: Two hundred and nine metastatic castration-resistant prostate cancer patients were enrolled. Median age was 73 years. Total 42.1% patients had pain, 14.4% had a performance status of two and 59.8% had a Gleason score ≥8. Total 31.1% had previously received ≥2 chemotherapies, 15.3 and 12% had been previously treated with abiraterone and cabazitaxel, respectively and 14.8% had received both.
RESULTS: Median progression-free survival and overall survival were 4.8 and 13.1 months, respectively. A prostate-specific antigen reduction ≥50% was observed in 49.1%. Total 32.7% abiraterone-pretreated patients achieved a biochemical response compared with 56% of abiraterone-naive patients.
CONCLUSION: Enzalutamide was safe and well tolerated. Its antitumor activity in abiraterone-pretreated patients was limited.
PATIENTS & METHODS: Two hundred and nine metastatic castration-resistant prostate cancer patients were enrolled. Median age was 73 years. Total 42.1% patients had pain, 14.4% had a performance status of two and 59.8% had a Gleason score ≥8. Total 31.1% had previously received ≥2 chemotherapies, 15.3 and 12% had been previously treated with abiraterone and cabazitaxel, respectively and 14.8% had received both.
RESULTS: Median progression-free survival and overall survival were 4.8 and 13.1 months, respectively. A prostate-specific antigen reduction ≥50% was observed in 49.1%. Total 32.7% abiraterone-pretreated patients achieved a biochemical response compared with 56% of abiraterone-naive patients.
CONCLUSION: Enzalutamide was safe and well tolerated. Its antitumor activity in abiraterone-pretreated patients was limited.
Oh WK, Cheng WY, Miao R, et al.
Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting.
Urol Oncol. 2018; 36(11):500.e1-500.e9 [PubMed] Related Publications
Real-world outcomes in patients with metastatic castration-resistant prostate cancer receiving second-line chemotherapy versus an alternative androgen receptor-targeted agent (ARTA) following early progression on a first-line ARTA in a US community oncology setting.
Urol Oncol. 2018; 36(11):500.e1-500.e9 [PubMed] Related Publications
OBJECTIVE: This retrospective observational study assessed if second-line chemotherapy vs. androgen receptor-targeted agents (ARTAs; abiraterone/enzalutamide) is associated with improved outcomes in metastatic castration-resistant prostate cancer (mCRCaP) patients who experience early progression on first-line ARTAs in a US community setting.
METHODS: Patients with mCRCaP (n = 345) who progressed ≤ 12 months after first-line ARTA and received second-line chemotherapy (docetaxel/cabazitaxel; n = 147) or ARTA (n = 198) between May 2011 and October 2014 were identified. Overall survival (OS), prostate-specific antigen (PSA) response and progression, and clinical response were compared for second-line chemotherapy vs. ARTA, using one-sided tests from second-line therapy initiation. Multivariate analyses were adjusted for: year, age, metastases, opioid use, Eastern Cooperative Oncology Group performance score, PSA, hemoglobin, alkaline phosphatase, lactate dehydrogenase (LDH), and albumin levels.
RESULTS: Patients receiving second-line chemotherapy vs. ARTA were younger (median: 74 vs. 79 years) and had a poorer prognosis in terms of PSA, LDH, alkaline phosphatase, albumin and hemoglobin levels, opioid use, and Halabi risk score (P < 0.05). Response rates were higher for chemotherapy vs. ARTA (PSA: adjusted odds ratio = 2.27, P = 0.005; clinical: adjusted odds ratio = 1.78; P = 0.020) and time to PSA progression was longer (adjusted hazard ratio [aHR] = 0.66; P = 0.010). A trend favored chemotherapy vs. ARTA for OS (aHR = 0.81, P = 0.148). Among patients with poor prognostic features, those receiving chemotherapy had significantly improved OS (Halabi intermediate-/high-risk score: aHR = 0.55, P = 0.009; hemoglobin < 11 g/dl: aHR = 0.41, P = 0.002; LDH > upper limit of normal: aHR = 0.18, P = 0.014; albumin < lower limit of normal: aHR = 0.42, P = 0.020).
CONCLUSION: Following early progression on first-line ARTA, second-line chemotherapy may be more beneficial in mCRCaP compared with second-line ARTA in patients with a poor prognosis.
METHODS: Patients with mCRCaP (n = 345) who progressed ≤ 12 months after first-line ARTA and received second-line chemotherapy (docetaxel/cabazitaxel; n = 147) or ARTA (n = 198) between May 2011 and October 2014 were identified. Overall survival (OS), prostate-specific antigen (PSA) response and progression, and clinical response were compared for second-line chemotherapy vs. ARTA, using one-sided tests from second-line therapy initiation. Multivariate analyses were adjusted for: year, age, metastases, opioid use, Eastern Cooperative Oncology Group performance score, PSA, hemoglobin, alkaline phosphatase, lactate dehydrogenase (LDH), and albumin levels.
RESULTS: Patients receiving second-line chemotherapy vs. ARTA were younger (median: 74 vs. 79 years) and had a poorer prognosis in terms of PSA, LDH, alkaline phosphatase, albumin and hemoglobin levels, opioid use, and Halabi risk score (P < 0.05). Response rates were higher for chemotherapy vs. ARTA (PSA: adjusted odds ratio = 2.27, P = 0.005; clinical: adjusted odds ratio = 1.78; P = 0.020) and time to PSA progression was longer (adjusted hazard ratio [aHR] = 0.66; P = 0.010). A trend favored chemotherapy vs. ARTA for OS (aHR = 0.81, P = 0.148). Among patients with poor prognostic features, those receiving chemotherapy had significantly improved OS (Halabi intermediate-/high-risk score: aHR = 0.55, P = 0.009; hemoglobin < 11 g/dl: aHR = 0.41, P = 0.002; LDH > upper limit of normal: aHR = 0.18, P = 0.014; albumin < lower limit of normal: aHR = 0.42, P = 0.020).
CONCLUSION: Following early progression on first-line ARTA, second-line chemotherapy may be more beneficial in mCRCaP compared with second-line ARTA in patients with a poor prognosis.
Hung SC, Wang SS, Li JR, et al.
Outcome of Patients with Metastatic Castration-resistant Prostate Cancer After PSA Progression with Abiraterone Acetate.
Anticancer Res. 2018; 38(9):5429-5436 [PubMed] Related Publications
Outcome of Patients with Metastatic Castration-resistant Prostate Cancer After PSA Progression with Abiraterone Acetate.
Anticancer Res. 2018; 38(9):5429-5436 [PubMed] Related Publications
BACKGROUND/AIM: The main purpose of this study was to evaluate the outcome of patients with prostate-specific antigen (PSA) progression after abiraterone acetate (AA) treatment for metastatic castration-resistant prostate cancer (mCRPC).
PATIENTS AND METHODS: Between 2012 and 2017, 83 patients with clinically-confirmed mCRPC previously treated with docetaxel with/without cabazitaxel followed by AA were included in this retrospective study. All patients received 1,000 mg AA with 5 or 10 mg prednisolone. Among them, 59 were eligible for this study based on PSA progression during the clinical course. Patients were divided into two groups, AA responders and AA non-responders according to previous PSA response to AA treatment. Overall survival and treatment response to subsequent therapy were analyzed.
RESULTS: The median overall survival of the 59 patients after AA-treated PSA progression was 12 (95% confidence interval(CI)=7.6-16.4) months and was longer in the AA-responding group compared to the non-responding group (25 vs. 8 months, p<0.001). The survival time after PSA progression on AA was longer in the AA-responsive group despite not being statistically different (13 vs. 7 months, p=0.126). Patients with AA treatment who received subsequent therapies after PSA progression had better overall survival than those without (18 vs. 4 months, p=0.003). In addition, there was a trend for better chemotherapy response in AA non-responders than AA responders, 62.5% (5/8) vs. 12.5% (1/8) respectively.
CONCLUSION: In our small retrospective patient experience, effective sequential treatments for patients with mCRPC provided overall survival benefit. Previous treatment response can act as a clinical predictor for subsequent treatment.
PATIENTS AND METHODS: Between 2012 and 2017, 83 patients with clinically-confirmed mCRPC previously treated with docetaxel with/without cabazitaxel followed by AA were included in this retrospective study. All patients received 1,000 mg AA with 5 or 10 mg prednisolone. Among them, 59 were eligible for this study based on PSA progression during the clinical course. Patients were divided into two groups, AA responders and AA non-responders according to previous PSA response to AA treatment. Overall survival and treatment response to subsequent therapy were analyzed.
RESULTS: The median overall survival of the 59 patients after AA-treated PSA progression was 12 (95% confidence interval(CI)=7.6-16.4) months and was longer in the AA-responding group compared to the non-responding group (25 vs. 8 months, p<0.001). The survival time after PSA progression on AA was longer in the AA-responsive group despite not being statistically different (13 vs. 7 months, p=0.126). Patients with AA treatment who received subsequent therapies after PSA progression had better overall survival than those without (18 vs. 4 months, p=0.003). In addition, there was a trend for better chemotherapy response in AA non-responders than AA responders, 62.5% (5/8) vs. 12.5% (1/8) respectively.
CONCLUSION: In our small retrospective patient experience, effective sequential treatments for patients with mCRPC provided overall survival benefit. Previous treatment response can act as a clinical predictor for subsequent treatment.
Thomas C, Brandt MP, Baldauf S, et al.
Docetaxel-rechallenge in castration-resistant prostate cancer: defining clinical factors for successful treatment response and improvement in overall survival.
Int Urol Nephrol. 2018; 50(10):1821-1827 [PubMed] Related Publications
Docetaxel-rechallenge in castration-resistant prostate cancer: defining clinical factors for successful treatment response and improvement in overall survival.
Int Urol Nephrol. 2018; 50(10):1821-1827 [PubMed] Related Publications
PURPOSE: The purpose of the study was to define clinical factors for successful treatment response and re-exposure to docetaxel in metastatic castration-resistant prostate cancer (mCRPC).
METHODS: An mCRPC database of patients receiving first-line docetaxel and rechallenge courses was established. Several clinical factors were evaluated for prediction of treatment response. Multivariate cox-regression analysis was used to define pre-treatment and treatment factors for survival.
RESULTS: Between 2005 and 2013, 94 patients with mCRPC were treated with docetaxel. Full data set and follow-up were available for 62 patients. Median follow-up was 84 m [interquartile range (IQR) 64-104 m]. Median biochemical progression-free survival (bPFS) and overall survival under docetaxel were 9 m (IQR 5-16 m) and 20 m (IQR 16-26 m), respectively. Partial PSA-response at first docetaxel-sequence (n = 62), rechallenge (n = 32), and third-sequence (n = 22) docetaxel was 48.4%, 31.6%, and 34.8%, respectively. Time from start of primary androgen deprivation to CRPC > 47 m was the only independent pre-treatment parameter to predict improved overall survival (Hazard Ratio 0.48, p = 0.015). Interestingly, there was a strong trend for improved overall survival in patients with high Gleason Score (Hazard Ratio 0.58; p = 0.08). Partial PSA-response at docetaxel-rechallenge (Hazard Ratio 0.31; p = 0.008) and treatment-free interval > 3 m (Hazard Ratio 3.49; p = 0.014) were the only independent predictive factors under taxane treatment for overall survival.
CONCLUSION: Despite novel hormonal drugs, docetaxel still plays an important role in the treatment of mCRPC. Patients with partial-PSA-response at rechallenge or a treatment-free interval > 3 m benefit most from docetaxel re-exposure.
METHODS: An mCRPC database of patients receiving first-line docetaxel and rechallenge courses was established. Several clinical factors were evaluated for prediction of treatment response. Multivariate cox-regression analysis was used to define pre-treatment and treatment factors for survival.
RESULTS: Between 2005 and 2013, 94 patients with mCRPC were treated with docetaxel. Full data set and follow-up were available for 62 patients. Median follow-up was 84 m [interquartile range (IQR) 64-104 m]. Median biochemical progression-free survival (bPFS) and overall survival under docetaxel were 9 m (IQR 5-16 m) and 20 m (IQR 16-26 m), respectively. Partial PSA-response at first docetaxel-sequence (n = 62), rechallenge (n = 32), and third-sequence (n = 22) docetaxel was 48.4%, 31.6%, and 34.8%, respectively. Time from start of primary androgen deprivation to CRPC > 47 m was the only independent pre-treatment parameter to predict improved overall survival (Hazard Ratio 0.48, p = 0.015). Interestingly, there was a strong trend for improved overall survival in patients with high Gleason Score (Hazard Ratio 0.58; p = 0.08). Partial PSA-response at docetaxel-rechallenge (Hazard Ratio 0.31; p = 0.008) and treatment-free interval > 3 m (Hazard Ratio 3.49; p = 0.014) were the only independent predictive factors under taxane treatment for overall survival.
CONCLUSION: Despite novel hormonal drugs, docetaxel still plays an important role in the treatment of mCRPC. Patients with partial-PSA-response at rechallenge or a treatment-free interval > 3 m benefit most from docetaxel re-exposure.
Nair RR, Piktel D, Geldenhuys WJ, Gibson LF
Combination of cabazitaxel and plicamycin induces cell death in drug resistant B-cell acute lymphoblastic leukemia.
Leuk Res. 2018; 72:59-66 [PubMed] Article available free on PMC after 15/10/2019 Related Publications
Combination of cabazitaxel and plicamycin induces cell death in drug resistant B-cell acute lymphoblastic leukemia.
Leuk Res. 2018; 72:59-66 [PubMed] Article available free on PMC after 15/10/2019 Related Publications
Bone marrow microenvironment mediated downregulation of BCL6 is critical for maintaining cell quiescence and modulating therapeutic response in B-cell acute lymphoblastic leukemia (ALL). In the present study, we have performed a high throughput cell death assay using BCL6 knockdown REH ALL cell line to screen a library of FDA-approved oncology drugs. In the process, we have identified a microtubule inhibitor, cabazitaxel (CAB), and a RNA synthesis inhibitor, plicamycin (PLI) as potential anti-leukemic agents. CAB and PLI inhibited cell proliferation in not only the BCL6 knockdown REH cell line, but also six other ALL cell lines. Furthermore, combination of CAB and PLI had a synergistic effect in inhibiting proliferation in a cytarabine-resistant (REH/Ara-C) ALL cell line. Use of nanoparticles for delivery of CAB and PLI demonstrated that the combination was very effective when tested in a co-culture model that mimics the in vivo bone marrow microenvironment that typically supports ALL cell survival and migration into protective niches. Furthermore, exposure to PLI inhibited SOX2 transcription and exposure to CAB inhibited not only Mcl-1 expression but also chemotaxis in ALL cells. Taken together, our study demonstrates the utility of concomitantly targeting different critical regulatory pathways to induce cell death in drug resistant ALL cells.
Laber DA, Chen MB, Jaglal M, et al.
Phase 2 Study of Cyclophosphamide, Etoposide, and Estramustine in Patients With Castration-Resistant Prostate Cancer.
Clin Genitourin Cancer. 2018; 16(6):473-481 [PubMed] Related Publications
Phase 2 Study of Cyclophosphamide, Etoposide, and Estramustine in Patients With Castration-Resistant Prostate Cancer.
Clin Genitourin Cancer. 2018; 16(6):473-481 [PubMed] Related Publications
BACKGROUND: There are no effective chemotherapies for patients with metastatic castration-resistant prostate cancer (mCRPC) whose disease has failed to respond to taxanes or patients who do not wish to receive intravenous drugs. We hypothesized that low doses of multiple medications with prolonged exposure would result in a high response rate and low toxicity.
PATIENTS AND METHODS: Patients with mCRPC were eligible for this phase 2 trial. The primary endpoint was a prostate-specific antigen decrease of more than 50%. CEE consisted of cyclophosphamide (50 mg/m
RESULTS: Fifty-two patients were enrolled and included in all evaluations. The prostate-specific antigen response rate was 46% in all patients, 53% in chemotherapy-naive subjects, and 31% after docetaxel chemotherapy. Thirty subjects had measurable lesions, 1 (3%) had complete response, 2 (7%) partial response, and 22 (73%) stable disease, for a clinical benefit of 83%. Sixty percent experienced an improvement in their performance status, and 65% reported improvement in their pain. The median overall survival was 18.6 months in all patients, 20.4 months in chemotherapy-naive patients and 11.3 months in patients whose disease progressed while receiving docetaxel therapy. Grade 3/4 treatment-related toxicities included 20% neutropenia, 10% thrombocytopenia, 10% deep-vein thrombosis, 8% anemia, 8% fatigue, 4% death, and 2% anorexia and stomatitis.
CONCLUSION: CEE was an all-oral, easy-to-administer, and effective triple-drug therapy for patients with mCRPC.
PATIENTS AND METHODS: Patients with mCRPC were eligible for this phase 2 trial. The primary endpoint was a prostate-specific antigen decrease of more than 50%. CEE consisted of cyclophosphamide (50 mg/m
RESULTS: Fifty-two patients were enrolled and included in all evaluations. The prostate-specific antigen response rate was 46% in all patients, 53% in chemotherapy-naive subjects, and 31% after docetaxel chemotherapy. Thirty subjects had measurable lesions, 1 (3%) had complete response, 2 (7%) partial response, and 22 (73%) stable disease, for a clinical benefit of 83%. Sixty percent experienced an improvement in their performance status, and 65% reported improvement in their pain. The median overall survival was 18.6 months in all patients, 20.4 months in chemotherapy-naive patients and 11.3 months in patients whose disease progressed while receiving docetaxel therapy. Grade 3/4 treatment-related toxicities included 20% neutropenia, 10% thrombocytopenia, 10% deep-vein thrombosis, 8% anemia, 8% fatigue, 4% death, and 2% anorexia and stomatitis.
CONCLUSION: CEE was an all-oral, easy-to-administer, and effective triple-drug therapy for patients with mCRPC.


 Docetaxel
Docetaxel