Skin cancer has always been and remains the leader among all tumors in terms of occurrence. One of the main factors responsible for skin cancer, natural and artificial UV radiation, causes the mutations that transform healthy cells into cancer cells. These mutations inactivate apoptosis, an event required to avoid the malignant transformation of healthy cells. Among these deadliest of cancers, melanoma and its 'younger sister', Merkel cell carcinoma, are the most lethal. The heavy toll of skin cancers stems from their rapid progression and the fact that they metastasize easily. Added to this is the difficulty in determining reliable margins when excising tumors and the lack of effective chemotherapy. Possibly the biggest problem posed by skin cancer is reliably detecting the extent to which cancer cells have spread throughout the body. The initial tumor is visible and can be removed, whereas metastases are invisible to the naked eye and much harder to eliminate. In our opinion, antisense oligonucleotides, which can be used in the form of targeted ointments, provide real hope as a treatment that will eliminate cancer cells near the tumor focus both before and after surgery.
Kimeswenger S, Mann U, Hoeller C, et al.
Vemurafenib impairs the repair of ultraviolet radiation-induced DNA damage.Melanoma Res. 2019; 29(2):134-144 [
PubMed]
Related Publications
Targeted therapy with the BRAF inhibitors vemurafenib and dabrafenib is an effective treatment regimen in patients with advanced melanoma carrying the BRAF V600E mutation. A common side effect is an enhanced rate of nonmelanoma skin cancer (NMSC). BRAF inhibition leads to a paradoxical enhanced MAPK signalling in BRAF wild-type cells, which might in part be responsible for the enhanced NMSC burden. It is known that disturbances of DNA repair result in an increased rate of NMSC. In the present study, it was investigated whether BRAF inhibitors might interfere with the repair of ultraviolet radiation-induced DNA damage in vitro. Epidermal keratinocytes of 11 Caucasian donors were treated with vemurafenib or dabrafenib and, 24 h later, exposed to ultraviolet A. DNA damage and repair capacity were analysed using south-western slot blot detecting cyclobutane pyrimidine dimers. Using PCR and DNA sequencing, RAS mutations and human papilloma virus genes were investigated. RNA expression was determined using a Gene Expression Chip and qRT-PCR. In 36% of keratinocytes, vemurafenib hampers the repair of ultraviolet A-induced DNA damage. No changes in DNA repair were observed with dabrafenib, indicating a possible substance-specific effect of vemurafenib. In none of the keratinocytes, pre-existing RAS mutations or human papilloma virus-associated DNA sequences were detected. The expression of the interferon-related damage resistance signature is decreased upon vemurafenib treatment in 36% of donors. The enhanced rate of NMSC in patients treated with vemurafenib might be partly related to a vemurafenib-driven impaired capacity for DNA repair.
Sequencing of whole cancer genomes has revealed an abundance of recurrent mutations in gene-regulatory promoter regions, in particular in melanoma where strong mutation hotspots are observed adjacent to ETS-family transcription factor (TF) binding sites. While sometimes interpreted as functional driver events, these mutations are commonly believed to be due to locally inhibited DNA repair. Here, we first show that low-dose UV light induces mutations preferably at a known ETS promoter hotspot in cultured cells even in the absence of global or transcription-coupled nucleotide excision repair (NER). Further, by genome-wide mapping of cyclobutane pyrimidine dimers (CPDs) shortly after UV exposure and thus before DNA repair, we find that ETS-related mutation hotspots exhibit strong increases in CPD formation efficacy in a manner consistent with tumor mutation data at the single-base level. Analysis of a large whole genome cohort illustrates the widespread contribution of this effect to recurrent mutations in melanoma. While inhibited NER underlies a general increase in somatic mutation burden in regulatory elements including ETS sites, our data supports that elevated DNA damage formation at specific genomic bases is at the core of the prominent promoter mutation hotspots seen in skin cancers, thus explaining a key phenomenon in whole-genome cancer analyses.
Farrell AW, Halliday GM, Lyons JG
Brahma deficiency in keratinocytes promotes UV carcinogenesis by accelerating the escape from cell cycle arrest and the formation of DNA photolesions.J Dermatol Sci. 2018; 92(3):254-263 [
PubMed]
Related Publications
BACKGROUND: Ultraviolet radiation (UVR) is the principal cause of keratinocyte skin cancers. Previous work found that levels of the chromatin remodelling protein, Brahma (Brm), are diminished during the progression from actinic keratoses to cutaneous squamous cell carcinomas in humans, and its loss in UV-irradiated mouse skin causes epidermal hyperplasia and increased tumour incidence.
METHODS: The skins of mice and mouse and human keratinocytes deficient in Brm were exposed to UVR and evaluated for cell cycle progression and DNA damage response.
OBJECTIVE: To identify the mechanisms by which loss of Brm contributes to UVR-induced skin carcinogenesis.
RESULTS: In both mouse keratinocytes and HaCaT cells, Brm deficiency led to an increased cell population growth following UVR exposure compared to cells with normal levels of Brm. Cell cycle analysis using a novel assay showed that Brm-deficient keratinocytes entered cell cycle arrest normally, but escaped from cell cycle arrest faster, enabling them to begin proliferating earlier. In mouse keratinocytes, Brm primarily affected accumulation in G
CONCLUSION: The loss of Brm in keratinocytes exposed to UVR enables them to resume proliferation while harboring DNA photolesions, leading to an increased fixation of mutations and, consequently, increased carcinogenesis.
Melanin, the pigment produced by specialized cells, melanocytes, is responsible for skin and hair color. Skin pigmentation is an important protective mechanism against the DNA damaging and mutagenic effects of solar ultraviolet radiation (UV). It is acknowledged that exposure to UV is the main etiological environmental factor for all forms of skin cancer, including melanoma. DNA repair capacity is another major factor that determines the risk for skin cancer. Human melanocytes synthesize eumelanin, the dark brown form of melanin, as well as pheomelanin, which is reddish-yellow in color. The relative rates of eumelanin and pheomelanin synthesis by melanocytes determine skin color and the sensitivity of skin to the drastic effects of solar UV. Understanding the complex regulation of melanocyte function and how it responds to solar UV has a huge impact on developing novel photoprotective strategies to prevent skin cancer, particularly melanoma, the most fatal form, which originates from melanocytes. This review provides an overview of the known differences in the photoprotective effects of eumelanin versus pheomelanin, how these two forms of melanin are regulated genetically and biochemically, and their impact on the DNA damaging effects of UV exposure. Additionally, this review briefly discusses the role of paracrine factors, focusing on α-melanocortin (α-melanocyte stimulating hormone; α-MSH), in regulating melanogenesis and the response of melanocytes to UV, and describes a chemoprevention strategy based on targeting the melanocortin 1 receptor (MC1R) by analogs of its physiological agonist α-MSH.
Helbig D, Mauch C, Buettner R, Quaas A
Immunohistochemical expression of melanocytic and myofibroblastic markers and their molecular correlation in atypical fibroxanthomas and pleomorphic dermal sarcomas.J Cutan Pathol. 2018; 45(12):880-885 [
PubMed]
Related Publications
BACKGROUND: Atypical fibroxanthomas (AFXs) and pleomorphic dermal sarcomas (PDSs) are UV-induced pleomorphic skin tumors with a non-specific immunoprofile. For that reason, exclusion of other dedifferentiated tumor entities by immunohistochemistry is still mandatory to avoid misdiagnosis.
METHODS: We determined the expression frequency of several melanocytic and myofibroblastic markers investigating 50 AFXs and PDSs.. Next-generation-sequencing (NGS) was performed in microphthalmia-associated transcription factor (MiTF)-expressing cases.
RESULTS: We identified one MiTF-expressing AFX and PDS, and two PDSs harboring single S100-positive dendritic cells whereas Melan A, HMB45, and SOX10 were negative. Calponin was moderately expressed by tumor giant cells in one PDS whereas h-caldesmon, desmin, and myogenin were not expressed in any of the AFXs or PDSs. The MiTF-positive AFX presented CDKN2A, OXA1L, and PDGFRA mutations whereas the PDS harbored a typical TP53 mutation. Both patients have not shown any tumor progression over the last 16 and 30 months.
CONCLUSION: Rarely, AFX and PDS express the melanocytic marker MiTF and/or the myofibroblastic marker calponin. In doubtful cases, using a panel of immunohistochemical markers helps to avoid misdiagnosis.
We have previously demonstrated that apigenin promotes the expression of antiangiogenic protein thrombospondin-1 (TSP1) via a mechanism driven by mRNA-binding protein HuR. Here, we generated a novel mouse model with whole-body THBS-1 gene knockout on SKH-1 genetic background, which allows studies of UVB-induced acute skin damage and carcinogenesis and tests TSP1 involvement in apigenin's anticancer effects. Apigenin significantly inhibited UVB-induced carcinogenesis in the wild-type (WT) animals but not in TSP1 KO (TKO) mice, suggesting that TSP1 is a critical component of apigenin's chemopreventive function in UVB-induced skin cancer. Importantly, TKO mice presented with the elevated cutaneous inflammation at baseline, which was manifested by increased inflammatory infiltrates (neutrophils and macrophages) and elevated levels of the two key inflammatory cytokines, IL-6 and IL-12. In agreement, maintaining normal TSP1 expression in the UVB-irradiated skin of WT mice using topical apigenin application caused a marked decrease of circulating inflammatory cytokines. Finally, TKO mice showed an altered population dynamics of the bone marrow myeloid progenitor cells (CD11b
Balupillai A, Nagarajan RP, Ramasamy K, et al.
Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin.Toxicol Appl Pharmacol. 2018; 352:87-96 [
PubMed]
Related Publications
Previously, we proved that caffeic acid (CA), a major dietary phenolic acid, prevents skin carcinogenesis by modulating inflammatory signaling in mouse skin. However, the actual mechanisms of CA against UVB (280-320 nm) induced photocarcinogenesis remains unclear. The present results confirms that CA significantly inhibits single UVB-induced CPDs formation, oxidative DNA damage, ROS generation and frequency of apoptotic cell death in human dermal fibroblasts (HDFa). Furthermore, CA prevents UVB-induced expression of PI3K and AKT kinases through activation of PTEN which subsequently promotes XPC dependant NER proteins such as XPC, XPE, TFIIH (p44) and ERCC1 in HDFa cells and mouse skin tissue. Further, CA directly activates PTEN through hydrogen bond and hydrophobic interactions. Taken together, these findings suggest that CA prevents UVB-induced photodamage through the activation of PTEN expression in human dermal fibroblasts and mouse skin.
Miwa S, Hoffman RM
Imaging DNA Repair After UV Irradiation Damage of Cancer Cells in GelfoamMethods Mol Biol. 2018; 1760:199-203 [
PubMed]
Related Publications
DNA damage repair in response to UVC irradiation was imaged in cancer cells growing in Gelfoam
BACKGROUND: Melanoma and prostate cancer may share risk factors. This study examined the association between serum PSA levels, which is a risk factor for prostate cancer, and variants in some melanoma-associated pigmentary genes.
METHODS: We studied participants, all aged 70+ years, in the Concord Health and Ageing in Men Project who had no history of prostatitis or received treatment for prostate disease (n = 1033). We genotyped variants in MC1R (rs1805007, rs1805008), ASIP (rs4911414, rs1015362), SLC45A2 (rs28777, rs16891982), IRF4 (rs12203592), TYRP1 (rs1408799), TYR (rs1126809, rs1042602), SLC24A2 (rs12896399), and OCA2 (rs7495174). Generalised linear dominant models with Poisson distribution, log link functions and robust variance estimators estimated adjusted percentage differences (%PSA) in mean serum PSA levels (ng/mL) between variant and wildtype (0%PSA = reference) genotypes, adjusting for age, body mass index, serum 25OHD levels and birth regions (Australia or New Zealand (ANZ), Europe or elsewhere).
RESULTS: Serum PSA levels were strongly associated with advancing age and birth regions: mean PSA levels were lower in Europe-born (-29.7%) and elsewhere-born (-11.7%) men than ANZ-born men (reference). Lower %PSA was observed in men with variants in SLC45A2: rs28777 (-19.6;95%CI: -33.5, -2.7), rs16891982 (-17.3;95%CI:-30.4,-1.7) than in wildtype men (reference). There were significant interactions between birth regions and PSA levels in men with variants in MC1R (rs1805007; p-interaction = 0.0001) and ASIP (rs4911414; p-interaction = 0.007). For these genes %PSA was greater in ANZ-born men and lower in Europe- and elsewhere-born men with the variant than it was in wildtype men. In a post hoc analysis, serum testosterone levels were increased in men with MC1R rs1805007 and serum dihydrotestosterone in men with ASIP rs1015362.
CONCLUSION: Men with SNPs in SLC45A2, who have less sun sensitive skin, have lower PSA levels. Men with SNPs in MC1R and ASIP, who have more sun sensitive skin, and were born in ANZ, have higher PSA levels. Androgens may modify these apparent associations of pigmentary genes and sun exposure with PSA levels.
IMPACT: PSA levels and possibly prostate cancer risk may vary with sun sensitivity and sun exposure, the effects of which might be modified by androgen levels.
Stark MS, Tan JM, Tom L, et al.
Whole-Exome Sequencing of Acquired Nevi Identifies Mechanisms for Development and Maintenance of Benign Neoplasms.J Invest Dermatol. 2018; 138(7):1636-1644 [
PubMed]
Related Publications
The melanoma transformation rate of an individual nevus is very low despite the detection of oncogenic BRAF or NRAS mutations in 100% of nevi. Acquired melanocytic nevi do, however, mimic melanoma, and approximately 30% of all melanomas arise within pre-existing nevi. Using whole-exome sequencing of 30 matched nevi, adjacent normal skin, and saliva we sought to identify the underlying genetic mechanisms for nevus development. All nevi were clinically, dermoscopically, and histopathologically documented. In addition to identifying somatic mutations, we found mutational signatures relating to UVR mirroring those found in cutaneous melanoma. In nevi we frequently observed the presence of the UVR mutation signature compared with adjacent normal skin (97% vs. 10%, respectively). Copy number aberration analysis showed that for nevi with copy number loss of tumor suppressor genes, this loss was balanced by loss of potent oncogenes. Moreover, reticular and nonspecific patterned nevi showed an increased (P < 0.0001) number of copy number aberrations compared with globular nevi. The mutation signature data generated in this study confirms that UVR strongly contributes to nevogenesis. Copy number changes reflect at a genomic level the dermoscopic differences of acquired melanocytic nevi. Finally, we propose that the balanced loss of tumor suppressor genes and oncogenes is a protective mechanism of acquired melanocytic nevi.
The growing incidence of melanoma is a serious public health issue that merits a thorough understanding of potential causative risk factors, which includes exposure to ultraviolet radiation (UVR). Though UVR has been classified as a complete carcinogen and has long been recognized for its ability to damage genomic DNA through both direct and indirect means, the precise mechanisms by which the UVA and UVB components of UVR contribute to the pathogenesis of melanoma have not been clearly defined. In this review, we therefore highlight recent studies that have addressed roles for UVA radiation in the generation of DNA damage and in modulating the subsequent cellular responses to DNA damage in melanocytes, which are the cell type that gives rise to melanoma. Recent research suggests that UVA not only contributes to the direct formation of DNA lesions but also impairs the removal of UV photoproducts from genomic DNA through oxidation and damage to DNA repair proteins. Moreover, the melanocyte microenvironment within the epidermis of the skin is also expected to impact melanomagenesis, and we therefore discuss several paracrine signaling pathways that have been shown to impact the DNA damage response in UV-irradiated melanocytes. Lastly, we examine how alterations to the immune microenvironment by UVA-associated DNA damage responses may contribute to melanoma development. Thus, there appear to be multiple avenues by which UVA may elevate the risk of melanoma. Protective strategies against excess exposure to UVA wavelengths of light therefore have the potential to decrease the incidence of melanoma. Environ. Mol. Mutagen. 59:438-460, 2018. © 2018 Wiley Periodicals, Inc.
Melanoma is a clinically heterogeneous disease, and current strategies for treatment of the primary tumour are based on pathological criteria alone. In the recent past, several DNA-sequencing and RNA-sequencing studies of primary and advanced melanoma samples have identified unique relationships between somatic mutations, genomic aberrations, and the genetic fingerprint of ultraviolet radiation (UVR). The recurrent patterns of genomic alterations reveal different disease pathways, drug targets and mechanisms limiting drug response. Here, we examine the known associations between the molecular categories of melanoma and the multidimensional UVR damage. Copyright © 2018 Pathological Society of Great Britain and Ireland. Published by John Wiley & Sons, Ltd.
Lehmann J, Seebode C, Martens MC, Emmert S
Xeroderma Pigmentosum - Facts and Perspectives.Anticancer Res. 2018; 38(2):1159-1164 [
PubMed]
Related Publications
Ultraviolet (UV)-induced DNA lesions are almost exclusively removed by the nucleotide excision repair (NER) pathway, which is essential for prevention of skin cancer development. Patients with xeroderma pigmentosum (XP) are extremely sun sensitive due to a genetic defect in components of the NER cascade. They present with first signs of premature skin aging at an early age, with a considerably increased risk of developing UV-induced skin cancer. XP belongs to the group of DNA repair defective disorders that are mainly diagnosed in the clinic and in hindsight confirmed at the molecular level. Unfortunately, there are no causative treatment options for this rare, autosomal-recessive disorder, emphasizing the importance of an early diagnosis. Subsequently, UV-protective measures such as the reduction of exposure to environmental UV and regular skin cancer screenings should be undertaken to substantially improve prognosis as well as the disease course.
Colebatch AJ, Scolyer RA
Trajectories of premalignancy during the journey from melanocyte to melanoma.Pathology. 2018; 50(1):16-23 [
PubMed]
Related Publications
A stepwise progression from melanocytic precursors to cutaneous melanoma is a well-established model, based on decades of careful observation and morphological analysis. The steps identified are benign melanocytic naevus, dysplastic naevus, 'radial growth phase' melanoma (including melanoma in situ) and 'vertical growth phase' melanoma (also termed tumourigenic melanoma). Recent genomic data have refined the understanding of the steps of melanoma development and their relationship to one another. These data support the existence of dysplastic naevi as distinct lesions; suggest the importance of clonal dynamics in the precursor steps of melanoma; and confirm the carcinogenic role of ultraviolet radiation throughout early melanoma development and progression. In this review, the steps of melanoma development and progression are summarised and discussed in the context of recent genomic studies. This new understanding of melanoma pathogenesis that has been facilitated through careful correlation of morphological and molecular features will allow the identification and development of robust biomarkers to assist in more accurate diagnosis and prognostication of melanocytic tumours.
Roy S
Impact of UV Radiation on Genome Stability and Human Health.Adv Exp Med Biol. 2017; 996:207-219 [
PubMed]
Related Publications
Gradual depletion of the atmospheric ozone layer during the past few years has increased the incidence of solar UV radiation specifically the UV-C on earth's surface is one of the major environmental concerns because of the harmful effects of this radiation in all forms of life. The solar UV radiation including the harmful wavelength range of UV-B (280-320 nm) represents a significant climatic stress for both animals and plants, causing damage to the fundamental biomolecules such as DNA, proteins and lipids, thus activating genotoxic stress and induces genome instability. When DNA absorbs UV-B light, energy from the photon causes covalent linkages to form between adjacent pyrimidine bases, creating photoproducts, primarily cyclobutane pyrimidine dimers (CPDs) and pyrimidine-6,4-pyrimidinone photoproduct (6,4PPs). Pyrimidine dimers create distortions in the DNA strands and therefore can inhibit DNA replication as well transcription. Lack of efficient repair of UV-induced DNA damage may induce the formation of DNA double stand breaks (DSBs), one of the serious forms of damage in DNA double helix, as well as oxidative damage. Unrepaired DSBs in the actively dividing somatic cells severely affect cell growth and development, finally results in loss of cell viability and development of various diseases, such as cancer in man.This chapter mainly highlights the incidence of solar UV-radiation on earth's surface along with the formation of major types of UV-induced DNA damage and the associated repair mechanisms as well as methods of detecting DNA damage and finally our present understanding on the impact on solar UV radiation on human health.
The sensitivity of Xeroderma pigmentosa (XP) patients to sunlight has spurred the discovery and genetic and biochemical analysis of the eight XP gene products (XPA-XPG plus XPV) responsible for this disorder. These studies also have served to elucidate the nucleotide excision repair (NER) process, especially the critical role played by the XPA protein. More recent studies have shown that NER also involves numerous other proteins normally employed in DNA metabolism and cell cycle regulation. Central among these is ataxia telangiectasia and Rad3-related (ATR), a protein kinase involved in intracellular signaling in response to DNA damage, especially DNA damage-induced replicative stresses. This review summarizes recent findings on the interplay between ATR as a DNA damage signaling kinase and as a novel ligand for intrinsic cell death proteins to delay damage-induced apoptosis, and on ATR's regulation of XPA and the NER process for repair of UV-induced DNA adducts. ATR's regulatory role in the cytosolic-to-nuclear translocation of XPA will be discussed. In addition, recent findings elucidating a non-NER role for XPA in DNA metabolism and genome stabilization at ds-ssDNA junctions, as exemplified in prematurely aging progeroid cells, also will be reviewed.
Orlow I, Shi Y, Kanetsky PA, et al.
The interaction between vitamin D receptor polymorphisms and sun exposure around time of diagnosis influences melanoma survival.Pigment Cell Melanoma Res. 2018; 31(2):287-296 [
PubMed]
Free Access to Full Article Related Publications
Evidence on the relationship between the vitamin D pathway and outcomes in melanoma is growing, although it is not always clear. We investigated the impact of measured levels of sun exposure at diagnosis on associations of vitamin D receptor gene (VDR) polymorphisms and melanoma death in 3336 incident primary melanoma cases. Interactions between six SNPs and a common 3'-end haplotype were significant (p < .05). These SNPs, and a haplotype, had a statistically significant association with survival among subjects exposed to high UVB in multivariable regression models and exerted their effect in the opposite direction among those with low UVB. SNPs rs1544410/BsmI and rs731236/TaqI remained significant after adjustment for multiple testing. These results suggest that the association between VDR and melanoma-specific survival is modified by sun exposure around diagnosis, and require validation in an independent study. Whether the observed effects are dependent or independent of vitamin D activation remains to be determined.
Bérubé R, Drigeard Desgarnier MC, Douki T, et al.
Persistence and Tolerance of DNA Damage Induced by Chronic UVB Irradiation of the Human Genome.J Invest Dermatol. 2018; 138(2):405-412 [
PubMed]
Related Publications
Exposure to solar UVB radiation leads to the formation of the highly mutagenic cyclobutane pyrimidine dimers (CPDs), the DNA damage responsible for mutations found in skin cancer. The frequency of CPD formation and the repair rate of those lesions are two important parameters to determine the probability of UVR-induced mutations. Previous work has shown that chronic irradiation with sublethal doses of UVB radiation (chronic low-dose UVB radiation) leads to the accumulation of residual CPD that persists over time. We have thus investigated the persistence, localization, and consequences on genome stability of those chronic low-dose UVB radiation-induced residual CPDs. We show that chronic low-dose UVB radiation-induced residual CPDs persist on DNA and are diluted via semiconservative replication. They are overrepresented in the heterochromatin and at the TT dipyrimidine sites, and they catalyze the incidence of sister chromatin exchange. Our results shed some light on the impact of chronic UVB radiation exposure on DNA, with a focus on residual CPDs, their distribution, and consequences.
Albibas AA, Rose-Zerilli MJJ, Lai C, et al.
Subclonal Evolution of Cancer-Related Gene Mutations in p53 Immunopositive Patches in Human Skin.J Invest Dermatol. 2018; 138(1):189-198 [
PubMed]
Related Publications
Normal sun-exposed skin contains numerous epidermal patches that stain positive for p53 protein (p53 immunopositive patches, PIPs), which are considered potential early precursors of skin cancer. Although the TP53 gene is mutated in many PIPs, it is unclear whether PIPs contain any other cancer-related mutations. Here we report that PIPs, predominantly <3,000 p53 immunopositive cells in size, within normal chronically exposed skin contain mutations in multiple genes that are mutated in cutaneous squamous cell cancers. These mutations in the PIPs were not detected within the non-PIP epidermis of corresponding normal chronically exposed skin. Although some of these genetic alterations are clonal in the PIPs, many of the mutations are subclonal within these lesions. Similar mutations are seen in later precancers (actinic keratoses and Bowen's disease). Our results demonstrate that PIPs in chronically exposed skin contain multiple mutations in cancer-related genes. In addition, the results indicate that the clonal evolution of mutations that are seen within later precancerous lesions and in established malignancy can also occur in PIPs within normal human skin.
Cutaneous malignant melanoma is an aggressive and potentially lethal form of skin cancer, particularly in its advanced and therapy-resistant stages, and the need for novel therapeutics and prognostic tools is acute. Incidence of melanoma has steadily increased over the past few decades, with exposure to the genome-damaging effects of ultraviolet radiation (UVR) well-recognized as a primary cause. A number of genetically-engineered mouse models (GEMMs) have been created that exhibit high incidence of spontaneous and induced forms of melanoma, and a select subset recapitulates its progression to aggressive and metastatic forms. These GEMMs hold considerable promise for providing insights into advanced stages of melanoma, such as potential therapeutic targets and prognostic markers, and as in vivo systems for testing of novel therapies. In this review, we summarize how the HGF/SF transgenic mouse has been used to reveal metastasis-regulating activity of four different genes (
Cumulative exposure to solar ultraviolet (SUV) irradiation is regarded as the major etiologic factor in the development of skin cancer. The activation of the MAPK cascades occurs rapidly and is vital in the regulation of SUV-induced cellular responses. The T-LAK cell-originated protein kinase (TOPK), an upstream activator of MAPKs, is heavily involved in inflammation, DNA damage, and tumor development. However, the chemopreventive and therapeutic effects of specific TOPK inhibitors in SUV-induced skin cancer have not yet been elucidated. In the current study, ADA-07, a novel TOPK inhibitor, was synthesized and characterized. Pull-down assay results, ATP competition, and
Kircik Md LH
A Novel Technology: Sunscreen With Actinic Damage Repair.J Drugs Dermatol. 2017; 16(5):s59-s60 [
PubMed]
Related Publications
.
Kunisada M, Hosaka C, Takemori C, et al.
CXCL1 Inhibition Regulates UVB-Induced Skin Inflammation and Tumorigenesis in Xpa-Deficient Mice.J Invest Dermatol. 2017; 137(9):1975-1983 [
PubMed]
Related Publications
Xeroderma pigmentosum complementation group A is a hereditary disease characterized by early onset of skin cancers and freckle-like pigmented maculae in sun-exposed sites. Although the etiology of the predisposition to UVR-induced skin tumors in xeroderma pigmentosum complementation group A is well investigated as a repair deficiency in UVR-induced DNA damage, the mechanism of exaggerated sunburn in patients with xeroderma pigmentosum complementation group A and whether UVR-induced inflammation relates to a skin tumor-prone phenotype remains to be elucidated. Using gene profiling of xeroderma pigmentosum complementation group A model mice, Xpa-deficient mice, we found that expression of CXCL1 in the skin and blood of Xpa-deficient mice increased significantly after UVB exposure over even a limited area compared with that of wild-type mice. We administered CXCL1 neutralizing antibody or the antioxidant agent, N-acetylcysteine, to Xpa-deficient mice after UVB irradiation and found significant suppression of blood levels of CXCL1, ear swelling and erythema, the hallmarks of inflammation and neutrophil chemotaxis. Xpa-deficient mice treated with chronic UVB exposure plus administration of CXCL1 neutralizing antibody or N-acetylcysteine yielded many fewer skin tumors compared with the control group. This indicates that the UVB-induced strong inflammatory response of Xpa-deficient mice plays a role in skin tumor development, which could be suppressed by regulating chemokines such as CXCL1.
To elucidate the complex molecular mechanisms underlying the adverse effects UV radiation (UVR) on skin homeostasis, we performed multi-omics studies to characterize UV-induced genetic and epigenetic changes. Human keratinocytes from a single donor treated with or without UVR were analyzed by RNA-seq, exome-seq, and H3K27ac ChIP-seq at 4 h and 72 h following UVR. Compared to the relatively moderate mutagenic effects of UVR, acute UV exposure induced substantial epigenomic and transcriptomic alterations, illuminating a previously underappreciated role of epigenomic and transcriptomic instability in skin pathogenesis. Integration of the multi-omics data revealed that UVR-induced transcriptional dysregulation of a subset of genes was attributable to either genetic mutations or global redistribution of H3K27ac. H3K27ac redistribution further led to the formation of distinctive super enhancers in UV-irradiated cells. Our analysis also identified several new UV target genes, including CYP24A1, GJA5, SLAMF7 and ETV1, which were frequently dysregulated in human squamous cell carcinomas, highlighting their potential as new molecular targets for prevention or treatment of UVR-induced skin cancers. Taken together, our concurrent multi-omics analyses provide new mechanistic insights into the complex molecular networks underlying UV photobiological effects, which have important implications in understanding its impact on skin homeostasis and pathogenesis.
Rawson RV, Johansson PA, Hayward NK, et al.
Unexpected UVR and non-UVR mutation burden in some acral and cutaneous melanomas.Lab Invest. 2017; 97(2):130-145 [
PubMed]
Related Publications
Ultraviolet radiation (UVR) mutagenesis causes nearly all cutaneous melanomas, however, since UVR signatures are largely absent in acral melanoma, as well as melanoma in sun-protected sites, the cause of these melanomas is unknown. Whole-genome sequencing data generated as part of the Australian Melanoma Genome Project was supplemented with a detailed histopathological assessment with the melanomas then classified as UVR or non-UVR related, based on their mutation signatures. The clinicopathological characteristics of melanomas with mutation signatures for their subtype were compared. Three (of 35=8.6%) acral melanomas, all clinically and pathologically verified as arising from acral or subungual locations, had predominant UVR mutation burden, whereas four (of 140=2.9%) cutaneous melanomas showed predominant non-UVR mutations. Among the acral melanomas, the few that were UVR dominant occurred in younger patients, had a higher mutation load and a proportion of mutation burden due to UVR, which was similar to that in melanomas from intermittently UVR-exposed skin. Acral melanomas with a UVR signature occurred most frequently in subungual sites and included tumors harboring BRAF or NF1 mutations. Cutaneous melanomas dominated by non-UVR signatures had lower mutation burdens counts and their primary tumors were thicker and had more mitoses than in other cutaneous melanomas. No histopathological features predicted UVR dominance in acral melanomas or non-UVR dominance in cutaneous melanomas. Our finding of acral/subungual melanomas with predominant UVR mutagenesis suggests that the nail plate and acral skin do not provide complete protection from UVR. Our data also confirm that cutaneous melanomas not caused by UVR are infrequent. Identifying where mutation burden is discordant with primary tumor anatomical site is likely to be clinically significant when determining treatment options for metastatic acral and cutaneous melanoma patients.
The suppression of the immune system by overexposure to ultraviolet (UV) radiation has been implicated in the initiation and progression of photocarcinogenesis. Numerous changes occur in the skin on UVB exposure, including the generation of inflammatory mediators, DNA damage, epigenetic modifications, and migration and functional alterations in the antigen-presenting dendritic cells. Although each of these alterations can elicit a cascade of events that have the potential to modulate immune sensitivity alone, there is emerging evidence that there is considerable crosstalk between these cascades. The development of an understanding of UV-induced changes in the skin that culminate in UV-induced immunosuppression, which has been implicated in the risk of nonmelanoma skin cancer, as a network of events has implications for the development of more effective chemopreventive strategies. In the current review article, we discuss the evidence of interactions between the various molecular targets and signaling mechanisms associated with UV-induced immunosuppression.
Ultraviolet (UV) radiation from sunlight represents a constant threat to genome stability by generating modified DNA bases such as cyclobutane pyrimidine dimers (CPD) and pyrimidine-pyrimidone (6-4) photoproducts (6-4PP). If unrepaired, these lesions can have deleterious effects, including skin cancer. Mammalian cells are able to neutralize UV-induced photolesions through nucleotide excision repair (NER). The NER pathway has multiple components including seven xeroderma pigmentosum (XP) proteins (XPA to XPG) and numerous auxiliary factors, including ataxia telangiectasia and Rad3-related (ATR) protein kinase and RCC1 like domain (RLD) and homologous to the E6-AP carboxyl terminus (HECT) domain containing E3 ubiquitin protein ligase 2 (HERC2). In this review we highlight recent data on the transcriptional and posttranslational regulation of NER activity.
Non-melanoma skin cancers (NMSC) are a growing problem given that solar ultraviolet B (UVB) radiation exposure is increasing most likely due to depletion of the atmospheric ozone layer and lack of adequate sun protection. Better preventive methods are urgently required to reduce UV-caused photodamage and NMSC incidence. Earlier, we have reported that silibinin treatment activates p53 and reduces photodamage and NMSC, both in vitro and in vivo; but whether silibinin exerts its protective effects primarily through p53 remains unknown. To address this question, we generated p53 heterozygous (p53
Singh A, Willems E, Singh A, et al.
Ultraviolet radiation-induced differential microRNA expression in the skin of hairless SKH1 mice, a widely used mouse model for dermatology research.Oncotarget. 2016; 7(51):84924-84937 [
PubMed]
Free Access to Full Article Related Publications
Cutaneous squamous cell carcinoma (cSCC) is the most common type of non-melanoma skin cancer that can metastasize. The major etiological factor associated with cSCC is Ultraviolet radiation (UVR) with a limited understanding of its molecular mechanism. It was hypothesized that there is a direct effect of UVR on modulation of microRNAs (miRNAs), a novel class of short noncoding RNAs which affects translation and stability of mRNAs. To test the hypothesis, the dorsal skin of the SKH1 mice (6-7 week old) was exposed to acute and chronic doses of UVR. In miRNA array profiling, we found differential expression (log fold change>1) of miR-25-5p between untreated and acute UVR treated (4kJ/m2) SKH1 mice skin. However, differential expression (>1 log fold) of miR-144-3p, miR-33-5p, miR-32-5p, miR-1983, miR-136-5p, miR-142-3p, miR-376a-3p, miR-142-5p, miR-3968, and miR-29b-3p was observed between untreated and chronically UVR treated mice skin. Differentially expressed selected miRNAs (miR-32-5p, miR-33-5p, miR-144-3p, and miR-376a-3p) were further validated in real time PCR using miRNA specific primers. Web based data mining, for the prediction of potential miRNA associated gene pathways in miRBase database revealed a link with important pathways (PI3K-Akt, MAPK, Wnt, transcriptional misregulation, and other oncogenic pathway) associated with cSCC. Furthermore, findings of PI3K-Akt pathway genes affected due to chronic UVR were confirmed using cDNA array.
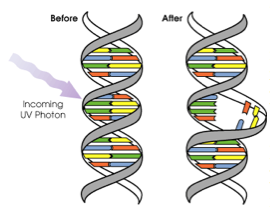 Ultraviolet (UV) photons (in sun light or from sun beds) can cause damage to DNA in different ways. The wavelength of the light determines how deeply the UV penetrates the skin. UVB (wavelength 280-320nm) is mainly absorbed by the epidermis (outer layer of skin) and can directly damage the DNA in skin cells. If not repaired properly, this damage can be a step in the development of skin cancer. UVA (wavelength 320-400nm) penetrates more deeply into the the lower epidermis and dermis (thick inner layer of skin), and cause harm, such as causing oxidative damage to skin cells.
Ultraviolet (UV) photons (in sun light or from sun beds) can cause damage to DNA in different ways. The wavelength of the light determines how deeply the UV penetrates the skin. UVB (wavelength 280-320nm) is mainly absorbed by the epidermis (outer layer of skin) and can directly damage the DNA in skin cells. If not repaired properly, this damage can be a step in the development of skin cancer. UVA (wavelength 320-400nm) penetrates more deeply into the the lower epidermis and dermis (thick inner layer of skin), and cause harm, such as causing oxidative damage to skin cells. Apoptosis
Apoptosis