Found this page useful?
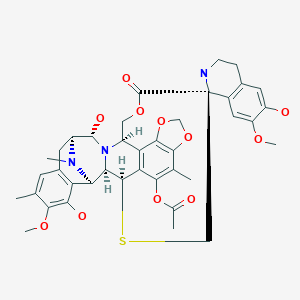
Trabectedin
 Web Resources: Trabectedin
Web Resources: Trabectedin Recent Research Publications
Recent Research PublicationsWeb Resources: Trabectedin (5 links)
Cancer Research UK
Macmillan Cancer Support
NHS Evidence
 Trabectedin - Substance Summary
Trabectedin - Substance Summary
PubChem
Irish Cancer Society
Recent Research Publications
Iwasaki J, Komori T, Nakagawa F, et al.
Schlafen11 Expression Is Associated With the Antitumor Activity of Trabectedin in Human Sarcoma Cell Lines.
Anticancer Res. 2019; 39(7):3553-3563 [PubMed] Related Publications
Schlafen11 Expression Is Associated With the Antitumor Activity of Trabectedin in Human Sarcoma Cell Lines.
Anticancer Res. 2019; 39(7):3553-3563 [PubMed] Related Publications
BACKGROUND/AIM: Trabectedin is a DNA-damaging agent and has been approved for the treatment of patients with advanced soft tissue sarcoma. Schlafen 11 (SLFN11) was identified as a dominant determinant of the response to DNA-damaging agents. The aim of the study was to clarify the association between SLFN11 expression and the antitumor activity of trabectedin.
MATERIALS AND METHODS: The antitumor activity of trabectedin was evaluated under different expression levels of SLFN11 regulated by RNA interference and CRISPR-Cas9 systems, and the combined antitumor activity of ataxia telangiectasia and Rad3-related protein kinase (ATR) inhibitor and trabectedin in sarcoma cell lines using in vitro a cell viability assay and in vivo xenograft models.
RESULTS: SLFN11-knockdown cell lines had a lower sensitivity to trabectedin, compared to parental cells. ATR inhibitor enhanced the antitumor activity of trabectedin in SLFN11-knockdown cells and in a SLFN11-knockout xenograft model.
CONCLUSION: SLFN11 expression might be a key factor in the antitumor activity of trabectedin.
MATERIALS AND METHODS: The antitumor activity of trabectedin was evaluated under different expression levels of SLFN11 regulated by RNA interference and CRISPR-Cas9 systems, and the combined antitumor activity of ataxia telangiectasia and Rad3-related protein kinase (ATR) inhibitor and trabectedin in sarcoma cell lines using in vitro a cell viability assay and in vivo xenograft models.
RESULTS: SLFN11-knockdown cell lines had a lower sensitivity to trabectedin, compared to parental cells. ATR inhibitor enhanced the antitumor activity of trabectedin in SLFN11-knockdown cells and in a SLFN11-knockout xenograft model.
CONCLUSION: SLFN11 expression might be a key factor in the antitumor activity of trabectedin.
Ishtiaq R, Naeem A, Ratnani I
Thoracic Liposarcoma In An End Stage Renal Disease Patient.
J Ayub Med Coll Abbottabad. 2019 Apr-Jun; 31(2):286-289 [PubMed] Related Publications
Thoracic Liposarcoma In An End Stage Renal Disease Patient.
J Ayub Med Coll Abbottabad. 2019 Apr-Jun; 31(2):286-289 [PubMed] Related Publications
Liposarcoma arising in the thoracic cavity is a rare entity. It is usually found in the retroperitoneal space and the extremities. No case of thoracic liposarcoma in a patient suffering from the end-stage renal disease has been reported in the literature. We herein present the first case of thoracic liposarcoma in a patient suffering from the end-stage renal disease. Metabolic disturbances, increased use of erythropoietin and increased diagnostic workup attributes to greater risk of cancer in patients suffering from renal failure. A chemotherapeutic drug, Trabectedin has been approved for advanced liposarcoma. Prognosis of such tumours depends on the size, location, and their histological subtype.
Chen Y, Xu L, Mayakonda A, et al.
Bromodomain and extraterminal proteins foster the core transcriptional regulatory programs and confer vulnerability in liposarcoma.
Nat Commun. 2019; 10(1):1353 [PubMed] Free Access to Full Article Related Publications
Bromodomain and extraterminal proteins foster the core transcriptional regulatory programs and confer vulnerability in liposarcoma.
Nat Commun. 2019; 10(1):1353 [PubMed] Free Access to Full Article Related Publications
Liposarcomas (LPSs) are a group of malignant mesenchymal tumors showing adipocytic differentiation. Here, to gain insight into the enhancer dysregulation and transcriptional addiction in this disease, we chart super-enhancer structures in both LPS tissues and cell lines. We identify a bromodomain and extraterminal (BET) protein-cooperated FUS-DDIT3 function in myxoid LPS and a BET protein-dependent core transcriptional regulatory circuitry consisting of FOSL2, MYC, and RUNX1 in de-differentiated LPS. Additionally, SNAI2 is identified as a crucial downstream target that enforces both proliferative and metastatic potentials to de-differentiated LPS cells. Genetic depletion of BET genes, core transcriptional factors, or SNAI2 mitigates consistently LPS malignancy. We also reveal a compelling susceptibility of LPS cells to BET protein degrader ARV-825. BET protein depletion confers additional advantages to circumvent acquired resistance to Trabectedin, a chemotherapy drug for LPS. Moreover, this study provides a framework for discovering and targeting of core oncogenic transcriptional programs in human cancers.
Miwa S, Yamamoto N, Hayashi K, et al.
Therapeutic Targets for Bone and Soft-Tissue Sarcomas.
Int J Mol Sci. 2019; 20(1) [PubMed] Free Access to Full Article Related Publications
Therapeutic Targets for Bone and Soft-Tissue Sarcomas.
Int J Mol Sci. 2019; 20(1) [PubMed] Free Access to Full Article Related Publications
Due to the rarity and heterogeneity of bone and soft-tissue sarcomas, investigation into molecular targets and new treatments has been particularly challenging. Although intensive chemotherapy and establishment of surgical procedures have improved the outcomes of patients with sarcoma, the curative rate of recurrent and metastatic sarcomas is still not satisfactory. Recent basic science research has revealed some of the mechanisms of progression and metastasis of malignancies including proliferation, apoptosis, angiogenesis, tumor microenvironment, migration, invasion, and regulation of antitumor immune systems. Based on these basic studies, new anticancer drugs, including pazopanib, trabectedin, eribulin, and immune checkpoint inhibitors have been developed and the efficacies and safety of the new drugs have been assessed by clinical trials. This review summarizes new molecular therapeutic targets and advances in the treatment for bone and soft tissue sarcomas.
Loria R, Giliberti C, Bedini A, et al.
Very low intensity ultrasounds as a new strategy to improve selective delivery of nanoparticles-complexes in cancer cells.
J Exp Clin Cancer Res. 2019; 38(1):1 [PubMed] Free Access to Full Article Related Publications
Very low intensity ultrasounds as a new strategy to improve selective delivery of nanoparticles-complexes in cancer cells.
J Exp Clin Cancer Res. 2019; 38(1):1 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: The possibility to combine Low Intensity UltraSound (LIUS) and Nanoparticles (NP) could represent a promising strategy for drugs delivery in tumors difficult to treat overcoming resistance to therapies. On one side the NP can carry drugs that specifically target the tumors on the other the LIUS can facilitate and direct the delivery to the tumor cells. In this study, we investigated whether Very Low Intensity UltraSound (VLIUS), at intensities lower than 120 mW/cm
METHODS: VLIUS at different intensities and exposure time were applied to tumor and normal cells to evaluate the efficiency in uptake of labeled human ferritin (HFt)-based NP, the delivery of NP complexed Firefly luciferase reported gene (lipoplex-LUC), and the tumor-killing of chemotherapeutic agent.
RESULTS: Specifically, we found that specific VLIUS intensity (120 mW/cm
CONCLUSIONS: Our data shed novel lights on the potential application of VLIUS for the design and development of novel therapeutic strategies aiming to efficiently deliver NP loaded cargos or anticancer drugs into more aggressive and unresponsive tumors niche.
METHODS: VLIUS at different intensities and exposure time were applied to tumor and normal cells to evaluate the efficiency in uptake of labeled human ferritin (HFt)-based NP, the delivery of NP complexed Firefly luciferase reported gene (lipoplex-LUC), and the tumor-killing of chemotherapeutic agent.
RESULTS: Specifically, we found that specific VLIUS intensity (120 mW/cm
CONCLUSIONS: Our data shed novel lights on the potential application of VLIUS for the design and development of novel therapeutic strategies aiming to efficiently deliver NP loaded cargos or anticancer drugs into more aggressive and unresponsive tumors niche.
Jones JD, Sinder BP, Paige D, et al.
Trabectedin Reduces Skeletal Prostate Cancer Tumor Size in Association with Effects on M2 Macrophages and Efferocytosis.
Neoplasia. 2019; 21(2):172-184 [PubMed] Free Access to Full Article Related Publications
Trabectedin Reduces Skeletal Prostate Cancer Tumor Size in Association with Effects on M2 Macrophages and Efferocytosis.
Neoplasia. 2019; 21(2):172-184 [PubMed] Free Access to Full Article Related Publications
Macrophages play a dual role in regulating tumor progression. They can either reduce tumor growth by secreting antitumorigenic factors or promote tumor progression by secreting a variety of soluble factors. The purpose of this study was to define the monocyte/macrophage population prevalent in skeletal tumors, explore a mechanism employed in supporting prostate cancer (PCa) skeletal metastasis, and examine a novel therapeutic target. Phagocytic CD68+ cells were found to correlate with Gleason score in human PCa samples, and M2-like macrophages (F4/80
Le Cesne A
Making the Best of Available Options for Optimal Sarcoma Treatment.
Oncology. 2018; 95 Suppl 1:11-20 [PubMed] Related Publications
Making the Best of Available Options for Optimal Sarcoma Treatment.
Oncology. 2018; 95 Suppl 1:11-20 [PubMed] Related Publications
For 35 years options for treating advanced soft tissue sarcoma (STS) were limited to doxorubicin, dacarbazine and ifosfamide. In 2007, trabectedin was approved. Since then, several other agents have become available and many more are in development, ushering in a new era in disease management. Considerable scope exists for improving outcomes of advanced STS through better trial design and improved patient care in everyday practice. After anthracycline failure, there are a range of treatment options and, increasingly, the choice of therapy is histology driven. Introduction of newer agents and optimising use of established agents such as trabectedin has led to an increase in overall survival of advanced STS patients. Optimising treatment with trabectedin is being achieved through more extensive experience in drug management, mainly associated with use in earlier lines and uninterrupted use until disease progression. Identification by next-generation sequencing of a significant proportion of cases of actionable mutations among patients with advanced STS suggests a move towards matched therapy in future. As the armamentarium of active agents in advanced sarcoma increases, so too will the challenge of selecting the right drug for the right patient at the right time, in accordance with the patient's lifestyle and wishes.
El Bairi K, Atanasov AG, Amrani M, Afqir S
The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery.
Biomed Pharmacother. 2019; 109:2492-2498 [PubMed] Related Publications
The arrival of predictive biomarkers for monitoring therapy response to natural compounds in cancer drug discovery.
Biomed Pharmacother. 2019; 109:2492-2498 [PubMed] Related Publications
Intrinsic or acquired drug resistance, adverse drug reactions and tumor heterogeneity between and within cancer patients limit the efficacy of clinical management of advanced cancers. To overcome these barriers, predictive biomarkers have recently emerged to guide medical oncologists in the selection of cancer patients who will respond to various anticancer treatments and to improve the toxicity to benefit ratio. Notably, targeted therapy has significantly benefited from these advances, but the application of predictive biomarkers have been a bit slower with some drugs derived from natural sources such as trabectedin, cabazitaxel and alvocidib. In this paper, we discuss some recent advances regarding the use of cancer biomarkers to predict efficacy of some selected natural compounds with a focus on human clinical studies.
Martin-Liberal J, Pérez E, García Del Muro X
Investigational therapies in phase II clinical trials for the treatment of soft tissue sarcoma.
Expert Opin Investig Drugs. 2019; 28(1):39-50 [PubMed] Related Publications
Investigational therapies in phase II clinical trials for the treatment of soft tissue sarcoma.
Expert Opin Investig Drugs. 2019; 28(1):39-50 [PubMed] Related Publications
INTRODUCTION: Soft-tissue sarcomas (STS) are a heterogeneous group of diseases that are characterized by a historic lack of active treatment options. However, several new drugs and indications have become available in recent years.
AREAS COVERED: This article reviews the most relevant phase II studies that utilize chemotherapy agents (aldoxorubicin, amrubicin, trabectedin alone or in combination with doxorubicin, and gemcitabine plus docetaxel), targeted therapies (Imatinib, dasatinib, regorafenib, tivozanib, palbociclib and selinexor), a combination of chemotherapy plus targeted therapies (fucusing on doxorubicin plus olaratumab) and immunotherapies (pembrolizumab, combination of nivolumab plus ipilimumab and adaptive cell therapy) in STS (other than gastrointestinal stromal tumors) (GIST) published from 2015. Some of these strategies are under further clinical development or will likely be assessed in future phase III studies.
EXPERT OPINION: A series of novel treatments have shown encouraging results in STS in recent years. The most important is the combination of the standard cytotoxic agent doxorubicin plus the platelet-derived growth factor receptor (PDGFR) inhibitor olaratumab, although definitive results from a phase III trial are expected. Immunotherapy has not been as successful in STS so far. However, further investigations are ongoing.
AREAS COVERED: This article reviews the most relevant phase II studies that utilize chemotherapy agents (aldoxorubicin, amrubicin, trabectedin alone or in combination with doxorubicin, and gemcitabine plus docetaxel), targeted therapies (Imatinib, dasatinib, regorafenib, tivozanib, palbociclib and selinexor), a combination of chemotherapy plus targeted therapies (fucusing on doxorubicin plus olaratumab) and immunotherapies (pembrolizumab, combination of nivolumab plus ipilimumab and adaptive cell therapy) in STS (other than gastrointestinal stromal tumors) (GIST) published from 2015. Some of these strategies are under further clinical development or will likely be assessed in future phase III studies.
EXPERT OPINION: A series of novel treatments have shown encouraging results in STS in recent years. The most important is the combination of the standard cytotoxic agent doxorubicin plus the platelet-derived growth factor receptor (PDGFR) inhibitor olaratumab, although definitive results from a phase III trial are expected. Immunotherapy has not been as successful in STS so far. However, further investigations are ongoing.
Nakano K, Takahashi S
Translocation-Related Sarcomas.
Int J Mol Sci. 2018; 19(12) [PubMed] Free Access to Full Article Related Publications
Translocation-Related Sarcomas.
Int J Mol Sci. 2018; 19(12) [PubMed] Free Access to Full Article Related Publications
Chromosomal translocations are observed in approximately 20% of soft tissue sarcomas (STS). With the advances in pathological examination technology, the identification of translocations has enabled precise diagnoses and classifications of STS, and it has been suggested that the presence of and differences in translocations could be prognostic factors in some translocation-related sarcomas. Most of the translocations in STS were not regarded as targets of molecular therapies until recently. However, trabectedin, an alkylating agent, has shown clinical benefits against translocation-related sarcoma based on a modulation of the transcription of the tumor's oncogenic fusion proteins. Many molecular-targeted drugs that are specific to translocations (e.g., anaplastic lymphoma kinase and tropomyosin kinase related fusion proteins) have emerged. The progress in gene technologies has allowed researchers to identify and even induce new translocations and fusion proteins, which might become targets of molecular-targeted therapies. In this review, we discuss the clinical significance of translocation-related sarcomas, including their diagnoses and targeted therapies.
Assi T, Kattan J, El Rassy E, et al.
A comprehensive review of the current evidence for trabectedin in advanced myxoid liposarcoma.
Cancer Treat Rev. 2019; 72:37-44 [PubMed] Related Publications
A comprehensive review of the current evidence for trabectedin in advanced myxoid liposarcoma.
Cancer Treat Rev. 2019; 72:37-44 [PubMed] Related Publications
Myxoid liposarcoma (MLS) is a rare mesenchymal tumor that constitutes 10-20% of all liposarcomas. MLS is a translocation-related sarcoma (TRS) related to the chromosomal translocation t(12:16) (q13:p11), producing the FUS-CHOP oncoprotein that constitutes one of the main targets of trabectedin in MLS patients. It is known to be chemosensitive namely to trabectedin in contrast to other soft tissue sarcomas. The efficacy of this agent in MLS have been demonstrated in different settings including treatment-naïve and pre-treated patients with both locally advanced and metastatic disease. However, the benefits of trabectedin in MLS are shadowed by the limited activity of this drug in other subtypes of sarcomas that are enrolled within the same trials. This prompted us to screen the medical literature for clinical data that evaluates the efficacy and safety of trabectedin in MLS. In this review, we will summarize the available evidence for the applicability of trabectedin in MLS.
Casado A, Callata HR, Manzano A, et al.
Trabectedin for reversing platinum resistance and resensitization to platinum in patients with recurrent ovarian cancer.
Future Oncol. 2019; 15(3):271-280 [PubMed] Related Publications
Trabectedin for reversing platinum resistance and resensitization to platinum in patients with recurrent ovarian cancer.
Future Oncol. 2019; 15(3):271-280 [PubMed] Related Publications
AIMS: We evaluated trabectedin in patients with platinum-resistant/refractory and partially platinum-sensitive recurrent ovarian cancer and the outcomes after reintroduction of platinum.
METHODS: Twenty-seven patients (platinum-resistant/refractory n = 24/PPS; n = 3) treated with trabectedin were retrospectively analyzed.
RESULTS: Trabectedin resulted in an objective response rate (ORR) of 18.2% with a 59.1% of disease control rate (ORR plus stable disease). The median progression-free and overall survival were 3.0 and 21.3 months, respectively. Subsequently, 17 patients were retreated with platinum and yield an ORR of 41.2% and DCR of 47.0%. The median progression-free and overall survival after platinum rechallenge were 5.0 and 14.7 months, respectively.
CONCLUSION: Our results suggest that trabectedin may contribute to resensitize tumor cells to platinum rechallenge.
METHODS: Twenty-seven patients (platinum-resistant/refractory n = 24/PPS; n = 3) treated with trabectedin were retrospectively analyzed.
RESULTS: Trabectedin resulted in an objective response rate (ORR) of 18.2% with a 59.1% of disease control rate (ORR plus stable disease). The median progression-free and overall survival were 3.0 and 21.3 months, respectively. Subsequently, 17 patients were retreated with platinum and yield an ORR of 41.2% and DCR of 47.0%. The median progression-free and overall survival after platinum rechallenge were 5.0 and 14.7 months, respectively.
CONCLUSION: Our results suggest that trabectedin may contribute to resensitize tumor cells to platinum rechallenge.
Shamai S, Merimsky O
Trabectedin for Advanced Soft Tissue Sarcoma: Ten Year Real-Life Perspective.
Isr Med Assoc J. 2018; 20(10):599-603 [PubMed] Related Publications
Trabectedin for Advanced Soft Tissue Sarcoma: Ten Year Real-Life Perspective.
Isr Med Assoc J. 2018; 20(10):599-603 [PubMed] Related Publications
BACKGROUND: Trabectedin is a marine-derived chemotherapy, which has received U.S. Food and Drug Administration approval for use in anthracycline-resistant advanced soft tissue sarcoma (STS), especially liposarcoma and leiomyosarcoma (L-sarcomas).
OBJECTIVES: To describe our 10 year real-life experience with trabectedin regarding safety and efficacy in a cohort of 86 patients.
METHODS: In our study cohort, 46.51% were diagnosed with liposarcoma and 43.02% with leiomyosarcoma. A total of 703 cycles of trabectedin were given, with a median of five cycles per patient (range 1-59). Median overall survival was 13.5 months for the whole cohort, 11 months for liposarcoma patients (range 1-63), and 15 months for leiomyosarcoma patients (range 1-35).
RESULTS: There was no statistically significant difference in progression free survival when stratified according to previous treatment lines given. Trabectedin exhibited a favorable safety profile, with only 22% requiring dose reductions. Grade 3 and higher toxicity was noted in 25% of the patients, mostly due to myelosuppression. There were no treatment-related deaths.
CONCLUSIONS: Trabectedin is a safe and effective drug for treating advanced STS. Our results reflect real-life data with patients receiving the drug as a third and even fourth line of treatment, or with a suboptimal performance status, yet achieving impressive clinical benefit rates and survival.
OBJECTIVES: To describe our 10 year real-life experience with trabectedin regarding safety and efficacy in a cohort of 86 patients.
METHODS: In our study cohort, 46.51% were diagnosed with liposarcoma and 43.02% with leiomyosarcoma. A total of 703 cycles of trabectedin were given, with a median of five cycles per patient (range 1-59). Median overall survival was 13.5 months for the whole cohort, 11 months for liposarcoma patients (range 1-63), and 15 months for leiomyosarcoma patients (range 1-35).
RESULTS: There was no statistically significant difference in progression free survival when stratified according to previous treatment lines given. Trabectedin exhibited a favorable safety profile, with only 22% requiring dose reductions. Grade 3 and higher toxicity was noted in 25% of the patients, mostly due to myelosuppression. There were no treatment-related deaths.
CONCLUSIONS: Trabectedin is a safe and effective drug for treating advanced STS. Our results reflect real-life data with patients receiving the drug as a third and even fourth line of treatment, or with a suboptimal performance status, yet achieving impressive clinical benefit rates and survival.
Esser M, Kloth C, Thaiss WM, et al.
CT-response patterns and the role of CT-textural features in inoperable abdominal/retroperitoneal soft tissue sarcomas treated with trabectedin.
Eur J Radiol. 2018; 107:175-182 [PubMed] Related Publications
CT-response patterns and the role of CT-textural features in inoperable abdominal/retroperitoneal soft tissue sarcomas treated with trabectedin.
Eur J Radiol. 2018; 107:175-182 [PubMed] Related Publications
PURPOSE: To evaluate CT patterns and textural features of soft tissue sarcomas following trabectedin therapy as well as their suitability for predicting therapeutic response.
MATERIAL AND METHODS: A total of 31 patients (18 female, 13 male; mean age, 58.0years; range, 38-79years) with sarcoma under trabectedin as a third-line therapy between October 2008 and July 2017 underwent baseline and follow-up contrast-enhanced CT. Response evaluation was based on modifiedCHOI-criteria and RECIST1.1, classified as partial response(PR), stable disease(SD), progressive disease(PD). For CT-texture analysis (CTTA), mean, entropy and uniformity of intensity/skewness/entropy of co-occurrence matrix (COM) and contrast of neighboring-grey-level-dependence-matrix (NGLDM) were calculated.
RESULTS: Following CHOI-criteria, 9 patients achieved PR, 10 SD and 12 PD. RECIST1.1. classified patients into 5 PR, 15 SD and 11 PD. A frequent (n = 6/31; 19.3%) pattern of response was tumor liquefaction. In responders differences in entropy of entropy-NGLDM(p = 0.028) and uniformity-NGLDM(p = 0.021), in non-responders entropy of average(p = 0.039), deviation(p = 0.04) and uniformity of deviation(p = 0.013) occured between baseline and follow-up. Mean intensity and average were higher when liquefication occured(p = 0.03; p = 0.02), whereas mean deviation was lower(p = 0.02) at baseline compared to other response patterns. Differences in mean(p = 0.023), entropy(p = 0.049) and uniformity(p = 0.023) of entropy-NGLDM were found between responders and non-responders at follow-up. For the mean of heterogeneity a cut-off value was calculated for prediction of response in baseline CTTA (0.12; sensitivity 89%; specificity 77%).
CONCLUSION: A frequent pattern of response to trabectedin was tumor liquefication being responsible for pseudoprogression, therefore modifiedCHOI should be preferred. Single CT-textural features can be used complementarily for prediction and monitoring response to trabectedin.
MATERIAL AND METHODS: A total of 31 patients (18 female, 13 male; mean age, 58.0years; range, 38-79years) with sarcoma under trabectedin as a third-line therapy between October 2008 and July 2017 underwent baseline and follow-up contrast-enhanced CT. Response evaluation was based on modifiedCHOI-criteria and RECIST1.1, classified as partial response(PR), stable disease(SD), progressive disease(PD). For CT-texture analysis (CTTA), mean, entropy and uniformity of intensity/skewness/entropy of co-occurrence matrix (COM) and contrast of neighboring-grey-level-dependence-matrix (NGLDM) were calculated.
RESULTS: Following CHOI-criteria, 9 patients achieved PR, 10 SD and 12 PD. RECIST1.1. classified patients into 5 PR, 15 SD and 11 PD. A frequent (n = 6/31; 19.3%) pattern of response was tumor liquefaction. In responders differences in entropy of entropy-NGLDM(p = 0.028) and uniformity-NGLDM(p = 0.021), in non-responders entropy of average(p = 0.039), deviation(p = 0.04) and uniformity of deviation(p = 0.013) occured between baseline and follow-up. Mean intensity and average were higher when liquefication occured(p = 0.03; p = 0.02), whereas mean deviation was lower(p = 0.02) at baseline compared to other response patterns. Differences in mean(p = 0.023), entropy(p = 0.049) and uniformity(p = 0.023) of entropy-NGLDM were found between responders and non-responders at follow-up. For the mean of heterogeneity a cut-off value was calculated for prediction of response in baseline CTTA (0.12; sensitivity 89%; specificity 77%).
CONCLUSION: A frequent pattern of response to trabectedin was tumor liquefication being responsible for pseudoprogression, therefore modifiedCHOI should be preferred. Single CT-textural features can be used complementarily for prediction and monitoring response to trabectedin.
Marth C
Trabectedin + pegylated liposomal doxorubicin in third-line treatment of platinum-sensitive relapsed ovarian cancer: a case study.
Expert Rev Anticancer Ther. 2018; 18(sup1):19-22 [PubMed] Related Publications
Trabectedin + pegylated liposomal doxorubicin in third-line treatment of platinum-sensitive relapsed ovarian cancer: a case study.
Expert Rev Anticancer Ther. 2018; 18(sup1):19-22 [PubMed] Related Publications
BACKGROUND: The challenges of managing relapsed ovarian cancer increase as more advanced lines of chemotherapy are achieved.
METHODS: A case study is presented to illustrate the complexities of selecting treatment in a patient with platinum-sensitive relapsed ovarian cancer and exposure to two previous lines of platinum-based chemotherapy.
RESULTS: In this clinical scenario, options include re-treatment with platinum-based chemotherapy or treatment with a nonplatinum single-agent or a nonplatinum combination. In this case, the nonplatinum combination of trabectedin + pegylated liposomal doxorubicin (PLD) was selected as the patient had limited platinum sensitivity (progression-free interval of 9 months), no BRCA mutation, and taking into account evidence that the regimen is effective and safe in the third line and beyond and may restore platinum sensitivity. After nine cycles of trabectedin + PLD, there was no evidence of disease. The patient was able to resume normal activities during therapy. Progression-free interval (PFI) was 17 months before disease progression. Subsequent platinum rechallenge produced a partial response.
CONCLUSION: Trabectedin + PLD may be an option for patients with platinum-sensitive relapsed ovarian cancer, including those who have received two or more previous lines of platinum.
METHODS: A case study is presented to illustrate the complexities of selecting treatment in a patient with platinum-sensitive relapsed ovarian cancer and exposure to two previous lines of platinum-based chemotherapy.
RESULTS: In this clinical scenario, options include re-treatment with platinum-based chemotherapy or treatment with a nonplatinum single-agent or a nonplatinum combination. In this case, the nonplatinum combination of trabectedin + pegylated liposomal doxorubicin (PLD) was selected as the patient had limited platinum sensitivity (progression-free interval of 9 months), no BRCA mutation, and taking into account evidence that the regimen is effective and safe in the third line and beyond and may restore platinum sensitivity. After nine cycles of trabectedin + PLD, there was no evidence of disease. The patient was able to resume normal activities during therapy. Progression-free interval (PFI) was 17 months before disease progression. Subsequent platinum rechallenge produced a partial response.
CONCLUSION: Trabectedin + PLD may be an option for patients with platinum-sensitive relapsed ovarian cancer, including those who have received two or more previous lines of platinum.
Ray-Coquard I
Management of relapsed ovarian cancer in routine clinical practice: a case study.
Expert Rev Anticancer Ther. 2018; 18(sup1):9-11 [PubMed] Related Publications
Management of relapsed ovarian cancer in routine clinical practice: a case study.
Expert Rev Anticancer Ther. 2018; 18(sup1):9-11 [PubMed] Related Publications
OBJECTIVE: In patients with recurrent ovarian cancer, there are certain situations where further surgery and/or next-line platinum-based chemotherapy is not feasible or is not the best option. Multidisciplinary teams have a key role in reviewing available options and selecting the most appropriate intervention.
METHODS: A case study of relapsed ovarian cancer illustrates some of the factors that shape decision-making and shows the potential of a non-platinum-based regimen in the context of limited platinum sensitivity.
RESULTS: Taking into account the patient's individual circumstances, many options were discarded by the tumor board. Further surgery was not recommended as initial surgery had been suboptimal. Platinum-based regimens incorporating bevacizumab were not indicated due to exposure to bevacizumab in the first-line setting. Other platinum-based regimens were not recommended in general, due to limited platinum sensitivity (relapse <12 months after previous platinum) and unacceptable toxicity. A decision was taken to treat with trabectedin + pegylated liposomal doxorubicin (PLD), which provided 15 months of disease control. Subsequent platinum rechallenge led to a complete response.
CONCLUSION: Second-line use of trabectedin + PLD in patients with limited platinum sensitivity may restore sensitivity at next platinum.
METHODS: A case study of relapsed ovarian cancer illustrates some of the factors that shape decision-making and shows the potential of a non-platinum-based regimen in the context of limited platinum sensitivity.
RESULTS: Taking into account the patient's individual circumstances, many options were discarded by the tumor board. Further surgery was not recommended as initial surgery had been suboptimal. Platinum-based regimens incorporating bevacizumab were not indicated due to exposure to bevacizumab in the first-line setting. Other platinum-based regimens were not recommended in general, due to limited platinum sensitivity (relapse <12 months after previous platinum) and unacceptable toxicity. A decision was taken to treat with trabectedin + pegylated liposomal doxorubicin (PLD), which provided 15 months of disease control. Subsequent platinum rechallenge led to a complete response.
CONCLUSION: Second-line use of trabectedin + PLD in patients with limited platinum sensitivity may restore sensitivity at next platinum.
Colombo N
Recurrent ovarian cancer 8 months after induction and bevacizumab consolidation: rationale for using trabectedin + pegylated liposomal doxorubicin in second line.
Expert Rev Anticancer Ther. 2018; 18(sup1):13-17 [PubMed] Related Publications
Recurrent ovarian cancer 8 months after induction and bevacizumab consolidation: rationale for using trabectedin + pegylated liposomal doxorubicin in second line.
Expert Rev Anticancer Ther. 2018; 18(sup1):13-17 [PubMed] Related Publications
OBJECTIVES: Ovarian cancer patients with relapse 6-12 months after last platinum treatment, who have received bevacizumab consolidation and are not BRCA mutant, represent a considerable therapeutic challenge.
METHODS: By means of illustrative case study, this review evaluates various treatment strategies for use at first relapse in an ovarian cancer patient with limited sensitivity to platinum.
RESULTS: Clinical parameters predictive of complete resection in secondary cytoreductive surgery include an Eastern Cooperative Oncology Group performance status of 0, no residual disease after first surgery, and <500 mL of ascites. Options for systemic therapy include platinum-based therapies, non-platinum combinations, and non-platinum single agents. The patient's circumstances suggested that the non-platinum combination of trabectedin + pegylated liposomal doxorubicin (PLD) would be most appropriate. Trabectedin + PLD may enhance response to the next platinum cycle and thus prolong survival although this hypothesis requires confirmation. At minimum, trabectedin + PLD provides additional time for patients to recover from previous platinum toxicity while receiving an effective treatment.
CONCLUSION: In recurrent ovarian cancer patients with expected suboptimal response to platinum, trabectedin + PLD may offer an active alternative, which differs from the first-line schedule, and may enhance the efficacy of subsequent platinum rechallenge.
METHODS: By means of illustrative case study, this review evaluates various treatment strategies for use at first relapse in an ovarian cancer patient with limited sensitivity to platinum.
RESULTS: Clinical parameters predictive of complete resection in secondary cytoreductive surgery include an Eastern Cooperative Oncology Group performance status of 0, no residual disease after first surgery, and <500 mL of ascites. Options for systemic therapy include platinum-based therapies, non-platinum combinations, and non-platinum single agents. The patient's circumstances suggested that the non-platinum combination of trabectedin + pegylated liposomal doxorubicin (PLD) would be most appropriate. Trabectedin + PLD may enhance response to the next platinum cycle and thus prolong survival although this hypothesis requires confirmation. At minimum, trabectedin + PLD provides additional time for patients to recover from previous platinum toxicity while receiving an effective treatment.
CONCLUSION: In recurrent ovarian cancer patients with expected suboptimal response to platinum, trabectedin + PLD may offer an active alternative, which differs from the first-line schedule, and may enhance the efficacy of subsequent platinum rechallenge.
Jones RL, Le Cesne A, Ibrahim T, et al.
Preserving quality of life as a key treatment goal in advanced soft tissue sarcomas.
Expert Rev Anticancer Ther. 2018; 18(12):1241-1248 [PubMed] Related Publications
Preserving quality of life as a key treatment goal in advanced soft tissue sarcomas.
Expert Rev Anticancer Ther. 2018; 18(12):1241-1248 [PubMed] Related Publications
INTRODUCTION: Health-related quality of life (HRQoL) is a patient-reported outcome that addresses patients' perceptions of symptoms across physical, emotional, cognitive and social domains. As HRQoL is currently rarely measured outside clinical trials in oncology, it must be inferred from patients' everyday performance during treatment. To gain insight into the HRQoL of advanced STS patients receiving palliative treatment in clinical practice, three case studies of patients treated with trabectedin are examined. Areas covered: The patient in Case 1 has maintained complete remission for more than 8 years after receiving nine cycles of second-line trabectedin followed by secondary surgery for recurrent myxoid liposarcoma, and was able to resume normal activities during trabectedin treatment. Case 2 describes 10 years' follow-up of a patient with myxoid liposarcoma who remains well after many lines of chemotherapy including extended use of trabectedin in the second line. The third case illustrates the feasibility of extending survival time in an elderly patient with metastatic leiomyosarcoma who was able to maintain a busy and active lifestyle while receiving second-line trabectedin. Expert commentary: Owing to its relatively benign safety profile, trabectedin frequently permits prolonged therapy and is generally well tolerated, often allowing patients to carry on with normal daily activities.
Grignani G, D'Ambrosio L, Pignochino Y, et al.
Trabectedin and olaparib in patients with advanced and non-resectable bone and soft-tissue sarcomas (TOMAS): an open-label, phase 1b study from the Italian Sarcoma Group.
Lancet Oncol. 2018; 19(10):1360-1371 [PubMed] Related Publications
Trabectedin and olaparib in patients with advanced and non-resectable bone and soft-tissue sarcomas (TOMAS): an open-label, phase 1b study from the Italian Sarcoma Group.
Lancet Oncol. 2018; 19(10):1360-1371 [PubMed] Related Publications
BACKGROUND: Trabectedin is an alkylating drug with a unique mechanism of action causing single-strand and double-strand DNA breaks that activate DNA damage-response pathways. Based on our preclinical data, we hypothesised that poly(ADP-ribose) polymerase 1 (PARP1) inhibitors might be an ideal partner of trabectedin and aimed to assess the safety, identify the recommended phase 2 dose, and explore preliminary signs of activity of trabectedin and olaparib combination treatment in patients with bone and soft-tissue sarcoma.
METHODS: We did an open-label, multicentre, phase 1b study, recruiting patients from the national Italian sarcoma network aged 18 years and older with histologically confirmed bone and soft-tissue sarcoma progressing after standard treatments with Eastern Cooperative Oncology Group performance status of 1 or less. In a classic 3 + 3 design, patients received a 24 h infusion of trabectedin on day 1 and olaparib orally twice a day in 21-day cycles across six dose levels (trabectedin 0·675-1·3 mg/m
FINDINGS: Between Nov 17, 2014, and Jan 30, 2017, of 54 patients assessed for eligibility, we enrolled 50 patients: 28 patients in the dose-escalation cohort and 22 patients in the dose-expansion cohort. Patients received a median of four cycles of treatment (IQR 2-6; range 1-17 [the patients who received the highest number of cycles are still on treatment]) with a median follow-up of 10 months (IQR 5-23). Considering all dose levels, the most common grade 3-4 adverse events were lymphopenia (32 [64%] of 50 patients), neutropenia (31 [62%]), thrombocytopenia (14 [28%]), anaemia (13 [26%]), hypophosphataemia (20 [40%]), and alanine aminotransferase concentration increase (9 [18%]). No treatment-related life-threatening adverse events or deaths occurred. One (2%) patient interrupted treatment without progression without reporting any specific toxicity. Observed dose-limiting toxicities were thrombocytopenia, neutropenia for more than 7 days, and febrile neutropenia. We selected intermediate dose level 4b (trabectedin 1·1 mg/m
INTERPRETATION: Trabectedin and olaparib in combination showed manageable toxicities at active dose levels for both drugs. Preliminary data on antitumour activity are encouraging. Two dedicated phase 2 studies are planned to assess activity of this combination in both ovarian cancer (EudraCT2018-000230-35) and soft-tissue sarcomas.
FUNDING: Italian Association for Cancer Research, Italian Sarcoma Group, Foundation for Research on Musculoskeletal and Rare Tumors, and Italian Ministry of Health.
METHODS: We did an open-label, multicentre, phase 1b study, recruiting patients from the national Italian sarcoma network aged 18 years and older with histologically confirmed bone and soft-tissue sarcoma progressing after standard treatments with Eastern Cooperative Oncology Group performance status of 1 or less. In a classic 3 + 3 design, patients received a 24 h infusion of trabectedin on day 1 and olaparib orally twice a day in 21-day cycles across six dose levels (trabectedin 0·675-1·3 mg/m
FINDINGS: Between Nov 17, 2014, and Jan 30, 2017, of 54 patients assessed for eligibility, we enrolled 50 patients: 28 patients in the dose-escalation cohort and 22 patients in the dose-expansion cohort. Patients received a median of four cycles of treatment (IQR 2-6; range 1-17 [the patients who received the highest number of cycles are still on treatment]) with a median follow-up of 10 months (IQR 5-23). Considering all dose levels, the most common grade 3-4 adverse events were lymphopenia (32 [64%] of 50 patients), neutropenia (31 [62%]), thrombocytopenia (14 [28%]), anaemia (13 [26%]), hypophosphataemia (20 [40%]), and alanine aminotransferase concentration increase (9 [18%]). No treatment-related life-threatening adverse events or deaths occurred. One (2%) patient interrupted treatment without progression without reporting any specific toxicity. Observed dose-limiting toxicities were thrombocytopenia, neutropenia for more than 7 days, and febrile neutropenia. We selected intermediate dose level 4b (trabectedin 1·1 mg/m
INTERPRETATION: Trabectedin and olaparib in combination showed manageable toxicities at active dose levels for both drugs. Preliminary data on antitumour activity are encouraging. Two dedicated phase 2 studies are planned to assess activity of this combination in both ovarian cancer (EudraCT2018-000230-35) and soft-tissue sarcomas.
FUNDING: Italian Association for Cancer Research, Italian Sarcoma Group, Foundation for Research on Musculoskeletal and Rare Tumors, and Italian Ministry of Health.
Liu W, Jiang Q, Zhou Y
Advances of systemic treatment for adult soft-tissue sarcoma.
Chin Clin Oncol. 2018; 7(4):42 [PubMed] Related Publications
Advances of systemic treatment for adult soft-tissue sarcoma.
Chin Clin Oncol. 2018; 7(4):42 [PubMed] Related Publications
Soft-tissue sarcoma (STS) is a group of rare but highly heterogeneous neoplasms. Systemic treatment with cytotoxic chemotherapy and targeted agents is one of the main therapeutic modalities in patients with unresectable or metastatic disease, while adjuvant and neoadjuvant chemotherapy for adult-type sarcomas remain controversial. Although an anthracycline (doxorubicin) and ifosfamide remain the cornerstone for chemotherapy, advances have been made recently to exceed its limited efficacy, other agents such as trabectedin, eribulin have been approved. In a recent study, the addition of platelet-derived growth factor receptor (PDGFR) antibody-olaratumab to doxorubicin resulted in prolongation of progression-free survival and overall survival, which really means a breakthrough for STS. There is more emerging evidence of different sensitivity to treatment for different histological subtypes, second-line treatment for advanced sarcoma is being increasingly driven by histology. Cytotoxic drugs such as dacarbazine, gemcitabine, and taxanes have shown moderate activity in specific subtypes. Tyrosine kinase inhibitors (TKIs), including pazopanib and anlotinib, appear to be the promising targeted therapies. Other signal pathway inhibitors as CDK4/CDK6 inhibitor, imatinib, mTOR inhibitors, ALK inhibitor has shown some preliminary effect that need to be verified in the future trials. Checkpoint inhibitors as anti-PD-1 and CTLA-4 monoclonal antibodies have been used as a single agent or in combination in the early clinical trials, while further research needs to focus on better patient selection and new combinational strategies. In this review, we aim to summarize the advances of chemotherapy, targeted therapy and immunotherapy in the management of STS.
Benton CB, Chien KS, Tefferi A, et al.
Safety and tolerability of lurbinectedin (PM01183) in patients with acute myeloid leukemia and myelodysplastic syndrome.
Hematol Oncol. 2019; 37(1):96-102 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
Safety and tolerability of lurbinectedin (PM01183) in patients with acute myeloid leukemia and myelodysplastic syndrome.
Hematol Oncol. 2019; 37(1):96-102 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
Trabectedin is an FDA-approved DNA minor groove binder that has activity against translocation-associated sarcomas. Lurbinectedin is a next-generation minor groove binder with preclinical activity against myeloid leukemia cells. A dose-finding phase 1 clinical trial was performed in patients with acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) with further assessment of safety and tolerability. Forty-two patients with relapsed/refractory AML/MDS received lurbinectedin administered as a 1-hour intravenous infusion in a 3 + 3 study design. Two dosing schedules were used: 3.5, 5, 7, or 6 mg on days 1 and 8 or 2, 3, 1, or 1.5 mg for 3 consecutive days on days 1 to 3. Three patients experienced dose-limiting toxicities of rhabdomyolysis (grade 4), hyperbilirubinemia (grade 3), and oral herpes (grade 3) with the day 1 and 8 schedule. Otherwise, adverse events mainly consisted of gastrointestinal manifestations (n = 11), febrile neutropenia/infections (n = 4), pulmonary toxicity (n = 2), and renal failure (n = 2). The most common laboratory abnormalities observed were an increase in creatinine (93%) and anemia, neutropenia, and thrombocytopenia (100%). Overall, 33 of 42 patients (79%) had reduction in blasts in peripheral blood or bone marrow. One patient achieved a partial response and 2 patients a morphologic leukemia-free state. Most (n = 30, 71%) were discontinued due to progressive disease. Early deaths occurred from disease-related causes that were not attributable to lurbinectedin. Four patients with a chromosome 11q21-23 abnormality had significantly greater bone marrow blast reduction than those without such abnormality, with decrease of 31 ± 14% (n = 4) vs 8 ± 8% (n = 16), respectively (P = .04). Overall, lurbinectedin was safe and tolerated using the schedules and dose levels tested. While no sustained remissions were observed, single-agent lurbinectedin was transiently leukemia suppressive for some patients.
Kiyuna T, Tome Y, Murakami T, et al.
Trabectedin arrests a doxorubicin-resistant PDGFRA-activated liposarcoma patient-derived orthotopic xenograft (PDOX) nude mouse model.
BMC Cancer. 2018; 18(1):840 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
Trabectedin arrests a doxorubicin-resistant PDGFRA-activated liposarcoma patient-derived orthotopic xenograft (PDOX) nude mouse model.
BMC Cancer. 2018; 18(1):840 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
BACKGROUND: Pleomorphic liposarcoma (PLPS) is a rare, heterogeneous and an aggressive variant of liposarcoma. Therefore, individualized therapy is urgently needed. Our recent reports suggest that trabectedin (TRAB) is effective against several patient-derived orthotopic xenograft (PDOX) mouse models. Here, we compared the efficacy of first-line therapy, doxorubicin (DOX), and TRAB in a platelet-derived growth factor receptor-α (PDGFRA)-amplified PLPS.
METHODS: We used a fresh sample of PLPS tumor derived from a 68-year-old male patient diagnosed with a recurrent PLPS. Subcutaneous implantation of tumor tissue was performed in a nude mouse. After three weeks of implantation, tumor tissues were isolated and cut into small pieces. To match the patient a PDGFRA-amplified PLPS PDOX was created in the biceps femoris of nude mice. Mice were randomized into three groups: Group 1 (G1), control (untreated); Group 2 (G2), DOX-treated; Group 3 (G3), TRAB-treated. Measurement was done twice a week for tumor width, length, and mouse body weight.
RESULTS: The PLPS PDOX showed resistance towards DOX. However, TRAB could arrest the PLPS (p < 0.05 compared to control; p < 0.05 compared to DOX) without any significant changes in body-weight.
CONCLUSIONS: The data presented here suggest that for the individual patient the PLPS PDOX model could specifically distinguish both effective and ineffective drugs. This is especially crucial for PLPS because effective first-line therapy is harder to establish if it is not individualized.
METHODS: We used a fresh sample of PLPS tumor derived from a 68-year-old male patient diagnosed with a recurrent PLPS. Subcutaneous implantation of tumor tissue was performed in a nude mouse. After three weeks of implantation, tumor tissues were isolated and cut into small pieces. To match the patient a PDGFRA-amplified PLPS PDOX was created in the biceps femoris of nude mice. Mice were randomized into three groups: Group 1 (G1), control (untreated); Group 2 (G2), DOX-treated; Group 3 (G3), TRAB-treated. Measurement was done twice a week for tumor width, length, and mouse body weight.
RESULTS: The PLPS PDOX showed resistance towards DOX. However, TRAB could arrest the PLPS (p < 0.05 compared to control; p < 0.05 compared to DOX) without any significant changes in body-weight.
CONCLUSIONS: The data presented here suggest that for the individual patient the PLPS PDOX model could specifically distinguish both effective and ineffective drugs. This is especially crucial for PLPS because effective first-line therapy is harder to establish if it is not individualized.
Gadducci A, Grosso F, Scambia G, et al.
A phase II randomised (calibrated design) study on the activity of the single-agent trabectedin in metastatic or locally relapsed uterine leiomyosarcoma.
Br J Cancer. 2018; 119(5):565-571 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
A phase II randomised (calibrated design) study on the activity of the single-agent trabectedin in metastatic or locally relapsed uterine leiomyosarcoma.
Br J Cancer. 2018; 119(5):565-571 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
BACKGROUND: Patients with recurrent/metastatic uterine leiomyosarcoma (U-LMS) have a dismal prognosis. This phase II study aims to evaluate trabectedin efficacy and safety in advanced U-LMS.
METHODS: Eligible patients had received ≥ one line of chemotherapy. Gemcitabine ± docetaxel naive patients were randomised to Arm A: trabectedin 1.3 mg/m
RESULTS: Overall, 126 patients entered Arm A (45 from randomisation and 81 directly) and 42 Arm B. Arm A patients characteristics: median age = 57; ≥2 previous chemotherapy lines = 37.4%; metastatic disease = 93%. The study met the condition for trabectedin activity: PFS-6 = 35.2% (95% CI: 26.2-45). No difference in PFS by the number of previous chemotherapy lines emerged. Median OS = 20.6 months (IQR: 8-36.4). In Arm B, the PFS-6 = 51.5% (95% CI: 33.5-69.2). No toxic deaths occurred. In Arm A, only 4 patients interrupted treatment for toxicity.
CONCLUSIONS: Trabectedin is active and well tolerated, retaining similar efficacy across one to three previous lines of chemotherapy.
METHODS: Eligible patients had received ≥ one line of chemotherapy. Gemcitabine ± docetaxel naive patients were randomised to Arm A: trabectedin 1.3 mg/m
RESULTS: Overall, 126 patients entered Arm A (45 from randomisation and 81 directly) and 42 Arm B. Arm A patients characteristics: median age = 57; ≥2 previous chemotherapy lines = 37.4%; metastatic disease = 93%. The study met the condition for trabectedin activity: PFS-6 = 35.2% (95% CI: 26.2-45). No difference in PFS by the number of previous chemotherapy lines emerged. Median OS = 20.6 months (IQR: 8-36.4). In Arm B, the PFS-6 = 51.5% (95% CI: 33.5-69.2). No toxic deaths occurred. In Arm A, only 4 patients interrupted treatment for toxicity.
CONCLUSIONS: Trabectedin is active and well tolerated, retaining similar efficacy across one to three previous lines of chemotherapy.
Loria R, Laquintana V, Bon G, et al.
HMGA1/E2F1 axis and NFkB pathways regulate LPS progression and trabectedin resistance.
Oncogene. 2018; 37(45):5926-5938 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
HMGA1/E2F1 axis and NFkB pathways regulate LPS progression and trabectedin resistance.
Oncogene. 2018; 37(45):5926-5938 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
Although the medical treatments of sarcoma have evolved in the last years, a significant portion of patients develops recurrence after therapies suggesting the need to identify novel targets to improve the treatments. By the use of patient-derived and established cell lines from liposarcoma, as well as specimens from patient biopsies, we found that HMGA1 is involved in the progression of dedifferentiated and myxoid liposarcoma. The immunohistochemical and RT-PCR analyses of 68 liposarcoma specimens revealed a significant high expression of HMGA1, at the protein and RNA levels, both in myxoid and dedifferentiated liposarcoma subtypes compared with differentiated ones. Loss- and gain-of-function experiments by HMGA1-specific depletion and overexpression in dedifferentiated and myxoid liposarcoma cells showed the contribution of this oncogenic factor in cell proliferation, motility, invasion, and drug resistance. The in vitro and in vivo treatment of myxoid liposarcoma with trabectedin, a drug with a potent anti-tumor activity, revealed downregulation of HMGA1, E2F1, and its-downstream targets, vimentin and ZEB1, indicating a critical role of trabectedin in inhibiting the mesenchymal markers of these tumors through the HMGA1/E2F1 axis. These data were also confirmed in patients' tumor biopsies being HMGA1, E2F1, and vimentin expression significantly reduced upon trabectedin therapy, administered as neo-adjuvant chemotherapy. Furthermore, trabectedin treatment inhibits in vitro NFkB pathway in mixoyd liposarcoma sensitive but not in resistant counterparts, and the inhibition of NFkB pathway re-sensitizes the resistant cells to trabectedin treatment. These data support the rational for combining NFkB inhibitors with trabectedin in liposarcoma patients, who have become resistant to the drug.
Ray-Coquard I, Serre D, Reichardt P, et al.
Options for treating different soft tissue sarcoma subtypes.
Future Oncol. 2018; 14(10s):25-49 [PubMed] Related Publications
Options for treating different soft tissue sarcoma subtypes.
Future Oncol. 2018; 14(10s):25-49 [PubMed] Related Publications
Management of soft tissue sarcoma is increasingly subtype-dependent. Surgery is recommended for uterine leiomyosarcoma, with trabectedin being the preferred option for advanced disease when the treatment goal is long-term tumor stabilization. Liposarcoma subgroups are characterized by distinctive morphologies and genetics, different patterns of disease progression and clinical behavior, and variable responses to treatment. Genetic analysis of sarcomas has provided insights into pathogenesis with potential for developing new molecular targets. At the cytogenetic level, soft tissue sarcomas are categorized into specific, balanced translocations and those due to massive chromosomal rearrangements. For subtypes such as undifferentiated sarcomas, angiosarcomas, alveolar soft part sarcomas and clear cell sarcomas, evidence is especially limited, although it is known that these tumors display markedly different sensitivities to chemotherapeutic and targeted agents.
Blay JY
Getting up-to-date in the management of soft tissue sarcoma.
Future Oncol. 2018; 14(10s):3-13 [PubMed] Related Publications
Getting up-to-date in the management of soft tissue sarcoma.
Future Oncol. 2018; 14(10s):3-13 [PubMed] Related Publications
Surgery (+ radiation therapy in selected cases) is standard treatment for adult-type localized soft tissue sarcoma (STS). Accumulating randomized clinical evidence also supports adjuvant chemotherapy as a treatment option, although this remains contentious. Doxorubicin (± ifosfamide) is the standard first-line systemic treatment for advanced STS; however, newer chemotherapeutic agents may improve outcomes achieved with single-agent doxorubicin. In a Phase II study, adding olaratumab to doxorubicin markedly improved overall survival. Agents for second- and further lines include trabectedin, which combines long-term tumor stabilization with good quality of life, and gemcitabine + docetaxel which can produce a marked clinical response although at the cost of high toxicity. Pazopanib, eribulin, aldoxorubicin and regorafenib are other options for use in advanced STS.
Guerriero JL
Macrophages: The Road Less Traveled, Changing Anticancer Therapy.
Trends Mol Med. 2018; 24(5):472-489 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
Macrophages: The Road Less Traveled, Changing Anticancer Therapy.
Trends Mol Med. 2018; 24(5):472-489 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
Macrophages are present in all vertebrate tissues and have emerged as multifarious cells with complex roles in development, tissue homeostasis, and disease. Macrophages are a major constituent of the tumor microenvironment, where they either promote or inhibit tumorigenesis and metastasis depending on their state. Successful preclinical strategies to target macrophages for anticancer therapy are now being evaluated in the clinic and provide proof of concept that targeting macrophages may enhance current therapies; however, clinical success has been limited. This review discusses the promise of targeting macrophages for anticancer therapy, yet highlights how much is unknown regarding their ontogeny, regulation, and tissue-specific diversity. Further work might identify subsets of macrophages within different tissues, which could reveal novel therapeutic opportunities for anticancer therapy.
Runnebaum IB, Reichert D, Ringsdorf U, et al.
Trabectedin plus pegylated liposomal doxorubicin (PLD) for patients with platinum-sensitive recurrent ovarian cancer: a prospective, observational, multicenter study.
J Cancer Res Clin Oncol. 2018; 144(6):1185-1195 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
Trabectedin plus pegylated liposomal doxorubicin (PLD) for patients with platinum-sensitive recurrent ovarian cancer: a prospective, observational, multicenter study.
J Cancer Res Clin Oncol. 2018; 144(6):1185-1195 [PubMed] Article available free on PMC after 01/02/2020 Related Publications
PURPOSE: The OVA-YOND study is the first prospective, non-interventional trial designed to evaluate trabectedin (1.1 mg/m
METHODS: Eligible patients were adults with platinum-sensitive ROC, pretreated with ≥ 1 platinum-containing regimen/s. The primary endpoint was to assess safety/tolerability of the combination.
RESULTS: Seventy-seven patients with platinum-sensitive relapse from 31 sites were evaluated. Patients received a median of 6 cycles (range 1-21) with 39 patients (50.6%) receiving ≥ 6 cycles. Median treatment duration was 4.2 months (range 0.7-18.8), mostly on an outpatient basis (88.3% of patients). Most common grade 3/4 trabectedin-related adverse events (AEs) were leukopenia (18.2%), neutropenia (15.6%), thrombocytopenia (9.1%), alanine (7.8%) and aspartate aminotransferase (6.5%) increase, and nausea/vomiting (5.2% each). Neutropenia (18.2%), leukopenia (15.6%), thrombocytopenia (10.4%), and nausea/vomiting (5.2% each) were the most frequent grade 3/4 PLD-related AEs. No deaths attributed to drug-related AEs or unexpected AEs occurred. Five patients (6.5%) had a complete response and 19 patients (24.7%) achieved a partial response for an objective response rate of 31.2% with median response duration of 6.25 months. Sixteen patients (20.8%) had disease stabilization for a disease control rate of 51.9%. Median progression-free survival was 6.3 months and median overall survival was 16.4 months.
CONCLUSION: Trabectedin plus PLD confer clinically meaningful benefit to pre-treated patients with platinum-sensitive ROC, being comparable to those previously observed in selected populations from clinical trials and with a manageable safety profile.
METHODS: Eligible patients were adults with platinum-sensitive ROC, pretreated with ≥ 1 platinum-containing regimen/s. The primary endpoint was to assess safety/tolerability of the combination.
RESULTS: Seventy-seven patients with platinum-sensitive relapse from 31 sites were evaluated. Patients received a median of 6 cycles (range 1-21) with 39 patients (50.6%) receiving ≥ 6 cycles. Median treatment duration was 4.2 months (range 0.7-18.8), mostly on an outpatient basis (88.3% of patients). Most common grade 3/4 trabectedin-related adverse events (AEs) were leukopenia (18.2%), neutropenia (15.6%), thrombocytopenia (9.1%), alanine (7.8%) and aspartate aminotransferase (6.5%) increase, and nausea/vomiting (5.2% each). Neutropenia (18.2%), leukopenia (15.6%), thrombocytopenia (10.4%), and nausea/vomiting (5.2% each) were the most frequent grade 3/4 PLD-related AEs. No deaths attributed to drug-related AEs or unexpected AEs occurred. Five patients (6.5%) had a complete response and 19 patients (24.7%) achieved a partial response for an objective response rate of 31.2% with median response duration of 6.25 months. Sixteen patients (20.8%) had disease stabilization for a disease control rate of 51.9%. Median progression-free survival was 6.3 months and median overall survival was 16.4 months.
CONCLUSION: Trabectedin plus PLD confer clinically meaningful benefit to pre-treated patients with platinum-sensitive ROC, being comparable to those previously observed in selected populations from clinical trials and with a manageable safety profile.
Touati N, Schöffski P, Litière S, et al.
European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Experience with Advanced/Metastatic Epithelioid Sarcoma Patients Treated in Prospective Trials: Clinical Profile and Response to Systemic Therapy.
Clin Oncol (R Coll Radiol). 2018; 30(7):448-454 [PubMed] Related Publications
European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Experience with Advanced/Metastatic Epithelioid Sarcoma Patients Treated in Prospective Trials: Clinical Profile and Response to Systemic Therapy.
Clin Oncol (R Coll Radiol). 2018; 30(7):448-454 [PubMed] Related Publications
AIMS: Epithelioid sarcoma is a soft tissue sarcoma associated with a high rate of local recurrence after wide resection and high incidence of distant metastasis. Little is known about the clinical course and response to systemic treatments in epithelioid sarcoma patients. We carried out a retrospective analysis of clinical data from epithelioid sarcoma patients to provide a reference for the design of future epithelioid sarcoma-specific studies.
PATIENTS AND METHODS: Data from patients with epithelioid sarcoma entered in prospective multi-sarcoma phase II/III trials were pooled: EORTC trial 62012 (doxorubicin versus doxorubicin/ifosfamide), 62043 (pazopanib), 62072 (pazopanib versus placebo) and 62091 (doxorubicin versus trabectedin). Patients had either a local or a centrally confirmed diagnosis of epithelioid sarcoma, had inoperable/metastatic disease at study entry and were eligible for the according trial. Response was assessed according to RECIST 1.1. Progression-free survival (PFS) and overall survival were calculated from date of entry.
RESULTS: Among 976 patients with advanced sarcomas, 27 epithelioid sarcoma patients (2.8%) were eligible for the analysis (17 men, median age at diagnosis 50 years, range 19-72). Eighteen (66.7%) received chemotherapy as first-line treatment (five doxorubicin, eight doxorubicin/ifosfamide, two pazopanib, three trabectedin) and nine (33.3%) received pazopanib as second line or later. The primary tumour was located in the lower extremity (n = 8; 29.6%), upper extremity (n = 5; 18.5%), retro/intra-abdominal (n = 4; 14.8%) and in other locations (n = 10; 37.0%). At entry, metastases were mainly found in lung (n = 17; 63%), lymph nodes (n = 9; 33.3%), bone (n = 8; 29.6%) and soft tissue (n = 7; 25.9%). The best response for first-line patients was four partial responses (22.2%), 10 stable disease (55.6%) and four progressive disease (22.2%). In subsequent lines, pazopanib achieved one partial response (11.1%), four stable disease (44.4%) and four progressive disease (44.4%). All patients but one progressed on treatment. The median PFS and overall survival were 3.8 (95% confidence interval 2.2-4.8) and 10.8 months (95% confidence interval 8.1-21.3), respectively. Five patients were still alive at the time of the according trial analysis.
CONCLUSION: With all limitations of such a rare disease and small data set, objective response and survival outcomes are similar in epithelioid sarcoma to non-selected sarcoma populations. The clinical testing of novel systemic treatments for epithelioid sarcoma remains an unmet medical need and a high priority.
PATIENTS AND METHODS: Data from patients with epithelioid sarcoma entered in prospective multi-sarcoma phase II/III trials were pooled: EORTC trial 62012 (doxorubicin versus doxorubicin/ifosfamide), 62043 (pazopanib), 62072 (pazopanib versus placebo) and 62091 (doxorubicin versus trabectedin). Patients had either a local or a centrally confirmed diagnosis of epithelioid sarcoma, had inoperable/metastatic disease at study entry and were eligible for the according trial. Response was assessed according to RECIST 1.1. Progression-free survival (PFS) and overall survival were calculated from date of entry.
RESULTS: Among 976 patients with advanced sarcomas, 27 epithelioid sarcoma patients (2.8%) were eligible for the analysis (17 men, median age at diagnosis 50 years, range 19-72). Eighteen (66.7%) received chemotherapy as first-line treatment (five doxorubicin, eight doxorubicin/ifosfamide, two pazopanib, three trabectedin) and nine (33.3%) received pazopanib as second line or later. The primary tumour was located in the lower extremity (n = 8; 29.6%), upper extremity (n = 5; 18.5%), retro/intra-abdominal (n = 4; 14.8%) and in other locations (n = 10; 37.0%). At entry, metastases were mainly found in lung (n = 17; 63%), lymph nodes (n = 9; 33.3%), bone (n = 8; 29.6%) and soft tissue (n = 7; 25.9%). The best response for first-line patients was four partial responses (22.2%), 10 stable disease (55.6%) and four progressive disease (22.2%). In subsequent lines, pazopanib achieved one partial response (11.1%), four stable disease (44.4%) and four progressive disease (44.4%). All patients but one progressed on treatment. The median PFS and overall survival were 3.8 (95% confidence interval 2.2-4.8) and 10.8 months (95% confidence interval 8.1-21.3), respectively. Five patients were still alive at the time of the according trial analysis.
CONCLUSION: With all limitations of such a rare disease and small data set, objective response and survival outcomes are similar in epithelioid sarcoma to non-selected sarcoma populations. The clinical testing of novel systemic treatments for epithelioid sarcoma remains an unmet medical need and a high priority.
Grisanti S, Cosentini D, Tovazzi V, et al.
Hepatoprotective effect of N-acetylcysteine in trabectedin-induced liver toxicity in patients with advanced soft tissue sarcoma.
Support Care Cancer. 2018; 26(8):2929-2935 [PubMed] Related Publications
Hepatoprotective effect of N-acetylcysteine in trabectedin-induced liver toxicity in patients with advanced soft tissue sarcoma.
Support Care Cancer. 2018; 26(8):2929-2935 [PubMed] Related Publications
PURPOSE: Trabectedin is one of the few active agents in soft tissue sarcoma (STS) but hepatotoxicity is frequent and represents a dose-limiting factor. Protective strategies aiming at counteracting this important side effect have a crucial clinical impact. Due to its antioxidant properties, N-acetylcysteine (NAC) has a recognized hepatoprotective effect and this provides the rationale for testing NAC in the management of trabectedin-induced hepatotoxicity.
METHODS: Patients with recurrent or metastatic soft tissue sarcoma, consecutively observed at our institution, who were considered eligible to trabectedin, received concomitant NAC if they had impaired hepatic or renal function at baseline or developed hepatotoxicity during treatment. The study aim was to retrospectively explore trabectedin administration in terms of number of cycles, mean dose, and dose intensity (DI) in patients who received NAC as compared with those who did not. Secondary end points were progression-free survival (PFS) and overall survival (OS).
RESULTS: A total number of 18 patients were enrolled in this study. Nine received NAC and nine did not. The median number of administered trabectedin cycles, mean trabectedin dose/cycles, and median DI was comparable in the two groups (p = 0.450, p = 0.534, and p = 0.450, respectively). The PFS and OS curves overlapped.
CONCLUSION: This explorative study suggests that NAC can have a hepatoprotective activity in patients receiving trabectedin allowing to maintain an adequate dose intensity and continuative administration in patients with impaired liver and renal function or developing treatment-induced hepatotoxicity. A prospective randomized trial is warranted.
METHODS: Patients with recurrent or metastatic soft tissue sarcoma, consecutively observed at our institution, who were considered eligible to trabectedin, received concomitant NAC if they had impaired hepatic or renal function at baseline or developed hepatotoxicity during treatment. The study aim was to retrospectively explore trabectedin administration in terms of number of cycles, mean dose, and dose intensity (DI) in patients who received NAC as compared with those who did not. Secondary end points were progression-free survival (PFS) and overall survival (OS).
RESULTS: A total number of 18 patients were enrolled in this study. Nine received NAC and nine did not. The median number of administered trabectedin cycles, mean trabectedin dose/cycles, and median DI was comparable in the two groups (p = 0.450, p = 0.534, and p = 0.450, respectively). The PFS and OS curves overlapped.
CONCLUSION: This explorative study suggests that NAC can have a hepatoprotective activity in patients receiving trabectedin allowing to maintain an adequate dose intensity and continuative administration in patients with impaired liver and renal function or developing treatment-induced hepatotoxicity. A prospective randomized trial is warranted.


 Soft Tissue Sarcomas
Soft Tissue Sarcomas