Nedaplatin
"A second-generation cisplatin analogue with antineoplastic activity. Containing a novel ring structure in which glycolate is bound to the platinum by a bidentate ligand, nedaplatin forms reactive platinum complexes that bind to nucelophillic groups in DNA, resulting in intrastrand and interstrand DNA cross-links, apoptosis and cell death. This agent appears to be less nephrotoxic and neurotoxic compared to both cisplatin and carboplatin." (NCI Thesaurus)
Found this page useful?
 Web Resources: Nedaplatin
Web Resources: Nedaplatin Latest Research Publications
Latest Research PublicationsWeb Resources: Nedaplatin (1 links)
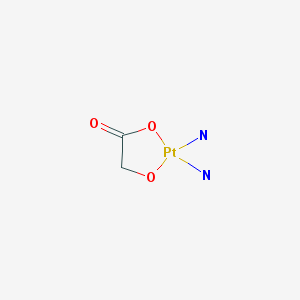
 Nedaplatin - Substance Summary
Nedaplatin - Substance Summary
PubChem
Latest Research Publications
Isaka T, Nakayama H, Yokose T, et al.
Platinum-Based Adjuvant Chemotherapy for Stage II and Stage III Squamous Cell Carcinoma of the Lung.
Ann Thorac Cardiovasc Surg. 2017; 23(1):19-25 [PubMed] Related Publications
Platinum-Based Adjuvant Chemotherapy for Stage II and Stage III Squamous Cell Carcinoma of the Lung.
Ann Thorac Cardiovasc Surg. 2017; 23(1):19-25 [PubMed] Related Publications
INTRODUCTION: The efficacy of platinum-based adjuvant chemotherapy (PBAC) for pathological stage II and stage III squamous cell carcinoma (SCC) of the lung was analyzed retrospectively.
MATERIALS AND METHODS: The prognoses of 94 patients with stage II and stage III SCC with or without PBAC (more than three courses of cisplatin-, carboplatin-, and nedaplatin-based adjuvant chemotherapy) were compared.
RESULTS: The mean observation period was 46.1 months. PBAC was not administered for the following reasons: 39 (55.7%) patients had comorbidities, 25 (35.7%) were older than 75 years, 19 (27.1%) patients underwent surgery before the approval of PBAC, and 3 (4.3%) patients could not continue PBAC (≤2 cycles) because of adverse events. PBAC patients (n = 24) were significantly younger than non-PBAC patients (n = 70; 66.3 vs 69.6 years old, respectively; p = 0.043). Disease-free survival (DFS) did not differ between PBAC and non-PBAC patients (55.0% and 67.1%, respectively; p = 0.266). PBAC patients tended to have worse overall survival (OS) than non-PBAC patients (56.1% and 70.2%, respectively; p = 0.138). PBAC was not prognostic for OS (hazard ratio (HR), 2.11; 95% confidence interval (CI), 0.82%-5.40%; p = 0.120).
CONCLUSION: PBAC did not improve the prognoses of patients with pathological stage II or stage III SCC in the single institution experience.
MATERIALS AND METHODS: The prognoses of 94 patients with stage II and stage III SCC with or without PBAC (more than three courses of cisplatin-, carboplatin-, and nedaplatin-based adjuvant chemotherapy) were compared.
RESULTS: The mean observation period was 46.1 months. PBAC was not administered for the following reasons: 39 (55.7%) patients had comorbidities, 25 (35.7%) were older than 75 years, 19 (27.1%) patients underwent surgery before the approval of PBAC, and 3 (4.3%) patients could not continue PBAC (≤2 cycles) because of adverse events. PBAC patients (n = 24) were significantly younger than non-PBAC patients (n = 70; 66.3 vs 69.6 years old, respectively; p = 0.043). Disease-free survival (DFS) did not differ between PBAC and non-PBAC patients (55.0% and 67.1%, respectively; p = 0.266). PBAC patients tended to have worse overall survival (OS) than non-PBAC patients (56.1% and 70.2%, respectively; p = 0.138). PBAC was not prognostic for OS (hazard ratio (HR), 2.11; 95% confidence interval (CI), 0.82%-5.40%; p = 0.120).
CONCLUSION: PBAC did not improve the prognoses of patients with pathological stage II or stage III SCC in the single institution experience.
Ohtake S, Kawahara T, Kasahara R, et al.
Pretreatment Neutrophil-to-Lymphocyte Ratio Can Predict the Prognosis in Bladder Cancer Patients Who Receive Gemcitabine and Nedaplatin Therapy.
Biomed Res Int. 2016; 2016:9846823 [PubMed] Free Access to Full Article Related Publications
Pretreatment Neutrophil-to-Lymphocyte Ratio Can Predict the Prognosis in Bladder Cancer Patients Who Receive Gemcitabine and Nedaplatin Therapy.
Biomed Res Int. 2016; 2016:9846823 [PubMed] Free Access to Full Article Related Publications
Introduction and Objectives. Neutrophil-to-lymphocyte ratio (NLR) has been suggested to be a simple marker of the systemic inflammatory response in critical care patients. We previously assessed the utility of NLR as a biomarker to predict tumor recurrence and cancer death in bladder cancer patients who underwent radical cystectomy. In this study, we evaluated the prognostic impact of NLR in bladder cancer patients who received gemcitabine and nedaplatin (GN) chemotherapy. Methods. A total of 23 patients who received GN chemotherapy for advanced bladder cancer were enrolled in this study. The cut-off point of NLR according to the sensitivity and specificity levels was derived from the area under receiver operator characteristics (AUROC) curve plotted for disease progression or overall mortality. Results. The NLR cut-off point was determined as 4.14 for both tumor progression and overall mortality. Median progression-free survival (PFS)/overall survival (OS) in the higher NLR group (NLR ≥ 4.14) and lower NLR group (NLR < 4.14) were 194/468 days versus 73/237 days, respectively. Kaplan-Meier analysis showed that higher NLR significantly correlated with poorer PFS (p = 0.011) and OS (p = 0.045). Conclusions. NLR may serve as a new biomarker to predict responses to GN-based chemotherapy in advanced bladder cancer patients and/or their prognosis.
Asaka S, Shimakawa T, Yamaguchi K, et al.
The Influence of Neoadjuvant Chemotherapy with Docetaxel, Nedaplatin and 5-Fluorouracil After Esophagectomy.
Anticancer Res. 2016; 36(11):6165-6171 [PubMed] Related Publications
The Influence of Neoadjuvant Chemotherapy with Docetaxel, Nedaplatin and 5-Fluorouracil After Esophagectomy.
Anticancer Res. 2016; 36(11):6165-6171 [PubMed] Related Publications
BACKGROUND: Neoadjuvant chemotherapy (NAC) with docetaxel, nedaplatin and 5-fluorouracil (5-FU) in esophageal cancer may adversely affect the postoperative clinical course following esophagectomy.
PATIENTS AND METHODS: We investigated the perioperative white blood cell count (WBC), C-reactive protein (CRP), serum albumin, body temperature (BT), heart rate (HR), respiratory rate (RR), water balance, partial pressure of oxygen in arterial blood (PaO2)/fraction of inspired oxygen (FiO2) ratio, postoperative complications and systemic inflammatory response syndrome (SIRS) in patients who underwent NAC or surgery alone (SA group).
RESULTS: In the NAC group, the preoperative WBC (p=0.015) and postoperative day (POD) 3 BT (p=0.049), as well as RR (p=0.037) were lower, whereas the POD 2 PaO2/FiO2 ratio was higher (p=0.047), compared to the SA group. No differences in the incidence of postoperative complications and SIRS were observed between the groups.
CONCLUSION: NAC using docetaxel, nedaplatin and 5-fluorouracil was tolerated and feasible in esophageal cancer.
PATIENTS AND METHODS: We investigated the perioperative white blood cell count (WBC), C-reactive protein (CRP), serum albumin, body temperature (BT), heart rate (HR), respiratory rate (RR), water balance, partial pressure of oxygen in arterial blood (PaO2)/fraction of inspired oxygen (FiO2) ratio, postoperative complications and systemic inflammatory response syndrome (SIRS) in patients who underwent NAC or surgery alone (SA group).
RESULTS: In the NAC group, the preoperative WBC (p=0.015) and postoperative day (POD) 3 BT (p=0.049), as well as RR (p=0.037) were lower, whereas the POD 2 PaO2/FiO2 ratio was higher (p=0.047), compared to the SA group. No differences in the incidence of postoperative complications and SIRS were observed between the groups.
CONCLUSION: NAC using docetaxel, nedaplatin and 5-fluorouracil was tolerated and feasible in esophageal cancer.
Hou J, Yu X, Hu Y, et al.
Value of intravoxel incoherent motion and dynamic contrast-enhanced MRI for predicting the early and short-term responses to chemoradiotherapy in nasopharyngeal carcinoma.
Medicine (Baltimore). 2016; 95(35):e4320 [PubMed] Free Access to Full Article Related Publications
Value of intravoxel incoherent motion and dynamic contrast-enhanced MRI for predicting the early and short-term responses to chemoradiotherapy in nasopharyngeal carcinoma.
Medicine (Baltimore). 2016; 95(35):e4320 [PubMed] Free Access to Full Article Related Publications
The aim of the study was to investigate the value of intravoxel incoherent motion diffusion-weighted magnetic resonance imaging (IVIM-DWI) and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) in predicting the early and short-term responses to chemoradiotherapy (CRT) in patients with nasopharyngeal carcinoma (NPC).Forty-three NPC patients underwent IVIM-DWI and DCE-MRI at baseline (pretreatment) and after the first cycle of induction chemotherapy (posttreatment). Based on whether locoregional lesions were identified, patients were divided into the residual and nonresidual groups at the end of CRT and into the good-responder and poor-responder groups 6 months after the end of CRT. The pretreatment and posttreatment IVIM-DWI parameters (ADC, D, D*, and f) and DCE-MRI parameters (K, Kep, and Ve) values and their percentage changes (Δ%) were compared between the residual and nonresidual groups and between the good-responder and poor-responder groups.None of perfusion-related parametric values derived from either DCE-MRI or IVIM-DWI showed significant differences either between the residual and nonresidual groups or between the good-responder and poor-responder groups. The nonresidual group exhibited lower pre-ADC, lower pre-D, and higher Δ%D values than did the residual group (all P <0.05). The good-responder group had lower pre-D and pre-ADC values than did the poor-responder group (both P <0.05). Based on receiver operating characteristic (ROC) curve analysis, pre-D had the highest area under the curve in predicting both the early and short-term responses to CRT for NPC patients (0.817 and 0.854, respectively).IVIM-DWI is more valuable than DCE-MRI in predicting the early and short-term response to CRT for NPC, and furthermore diffusion-related IVIM-DWI parameters (pre-ADC, pre-D, and Δ%D) are more powerful than perfusion-related parameters derived from both IVIM-DWI and DCE-MRI.
Hu W, Fang J, Nie J, et al.
Efficacy and safety of extended use of platinum-based doublet chemotherapy plus endostatin in patients with advanced nonsmall cell lung cancer.
Medicine (Baltimore). 2016; 95(28):e4183 [PubMed] Free Access to Full Article Related Publications
Efficacy and safety of extended use of platinum-based doublet chemotherapy plus endostatin in patients with advanced nonsmall cell lung cancer.
Medicine (Baltimore). 2016; 95(28):e4183 [PubMed] Free Access to Full Article Related Publications
The aim of this study was to investigate the efficacy and safety of the extended use of platinum-based doublet chemotherapy (PT-DC) plus endostatin in patients with advanced nonsmall cell lung cancer (NSCLC).We performed a retrospective analysis of 200 newly diagnosed advanced NSCLC patients who had received at least 1 cycle of endostatin plus PT-DC between September 2009 and November 2014. Of these patients, 155 received 4 or more cycles of therapy (the extended therapy group), while 45 received less than 4 cycles of therapy (the control group). Clinical tumor responses, progression-free survival (PFS), overall survival (OS), and toxicity profiles were recorded and retrospectively analyzed.In the extended therapy group, 67 patients (43.2%) achieved a best overall response rate of partial response (PR), while in the control group, 13 patients (28.9%) had a best overall response rate of PR. After a median follow-up of 15.9 months, the median PFS and OS were 8.0 and 23.1 months in the extended arm and 5.8 and 14.0 months in the control arm, respectively. There were statistically significant differences in median PFS and OS between these 2 arms. Hematologic and gastrointestinal toxicities occurred more frequently in the extended therapy group, but no statistically significant difference was detected in grade 3 to 4 toxicities overall between these 2 groups.In conclusion, extended treatment using endostatin combined with PT-DC can provide additional survival benefits and satisfactory toxicity profiles in previously untreated patients with NSCLC, which merits further evaluation in a larger prospective study.
Uchinami Y, Myojin M, Takahashi H, et al.
Prognostic factors in clinical T1N0M0 thoracic esophageal squamous cell carcinoma invading the muscularis mucosa or submucosa.
Radiat Oncol. 2016; 11:84 [PubMed] Free Access to Full Article Related Publications
Prognostic factors in clinical T1N0M0 thoracic esophageal squamous cell carcinoma invading the muscularis mucosa or submucosa.
Radiat Oncol. 2016; 11:84 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Multimodality treatment is widely performed for clinical T1N0M0 (UICC-TNM classification, 7th edition) thoracic esophageal squamous cell carcinoma (ESCC), but available articles regarding treatment results are limited. This study assessed the outcomes of clinical T1N0M0 thoracic ESCC invading the muscularis mucosa (MM) or submucosa (SM) treated with radiotherapy (RT) or chemoradiotherapy (CRT).
METHODS: We retrospectively reviewed the medical charts of 90 patients with clinical T1N0M0 thoracic ESCC treated with RT or CRT in our hospital in 2004-2011. Of these 90 patients, we analyzed the cases of 71 patients who met our inclusion criteria. All 71 patients had MM or SM cancer. In the 47 patients treated with CRT, the chemotherapy regimen of 5-fluorouracil (5-FU) plus cisplatin (CDDP) was used for 46 patients and 5-FU and nedaplatin was used for one patient. Forty-five patients underwent endoscopic resection (ER) followed by RT or CRT as an additional treatment. Elective nodal irradiation (ENI) was used in 39 patients. For all analyses, statistical significance was defined as 0.05, and the Bonferroni correction was used for the multivariate analysis.
RESULTS: The median age was 70 years (range 47-84). With a median follow-up of 43.6 months (range 1.5-124.2), the 5-year overall survival (OS), disease-specific survival (DSS) and disease-free survival (DFS) rates were 64.0, 72.8 and 50.0 %, respectively. The multivariate analysis showed that performance status (PS) was an independent prognostic factors for DSS and DFS (DSS, p < 0.001; DFS, p < 0.001). Chemotherapy in addition to RT showed a trend for better DSS (p = 0.032) but was not significant following Bonferroni correction. ER and ENI were not significant predictive factors for DSS and DFS.
CONCLUSIONS: PS was an independent prognostic factor for DSS and DFS. ER and ENI had no significant relationship with DSS or DFS. The present results may be helpful in treatment decisions for clinical T1N0M0 thoracic ESCC.
METHODS: We retrospectively reviewed the medical charts of 90 patients with clinical T1N0M0 thoracic ESCC treated with RT or CRT in our hospital in 2004-2011. Of these 90 patients, we analyzed the cases of 71 patients who met our inclusion criteria. All 71 patients had MM or SM cancer. In the 47 patients treated with CRT, the chemotherapy regimen of 5-fluorouracil (5-FU) plus cisplatin (CDDP) was used for 46 patients and 5-FU and nedaplatin was used for one patient. Forty-five patients underwent endoscopic resection (ER) followed by RT or CRT as an additional treatment. Elective nodal irradiation (ENI) was used in 39 patients. For all analyses, statistical significance was defined as 0.05, and the Bonferroni correction was used for the multivariate analysis.
RESULTS: The median age was 70 years (range 47-84). With a median follow-up of 43.6 months (range 1.5-124.2), the 5-year overall survival (OS), disease-specific survival (DSS) and disease-free survival (DFS) rates were 64.0, 72.8 and 50.0 %, respectively. The multivariate analysis showed that performance status (PS) was an independent prognostic factors for DSS and DFS (DSS, p < 0.001; DFS, p < 0.001). Chemotherapy in addition to RT showed a trend for better DSS (p = 0.032) but was not significant following Bonferroni correction. ER and ENI were not significant predictive factors for DSS and DFS.
CONCLUSIONS: PS was an independent prognostic factor for DSS and DFS. ER and ENI had no significant relationship with DSS or DFS. The present results may be helpful in treatment decisions for clinical T1N0M0 thoracic ESCC.
Wang ZL, Chen Y, Li XT, et al.
Potential of Baseline Computed Tomography to Predict Long-Term Survival of Patients With Locally Advanced Esophageal Cancer Treated With Preoperative Chemotherapy: A Retrospective Cohort Study.
Medicine (Baltimore). 2016; 95(18):e3583 [PubMed] Free Access to Full Article Related Publications
Potential of Baseline Computed Tomography to Predict Long-Term Survival of Patients With Locally Advanced Esophageal Cancer Treated With Preoperative Chemotherapy: A Retrospective Cohort Study.
Medicine (Baltimore). 2016; 95(18):e3583 [PubMed] Free Access to Full Article Related Publications
In this study, we evaluated the efficacy of baseline computed tomography (CT) signs and postoperative TN stages on survival of patients with advanced esophageal squamous cell carcinoma with preoperative chemotherapy. Consecutive patients (n = 130) with preoperative chemotherapy and radical esophagectomy from January 2006 to December 2011 were enrolled in this study retrospectively. Pathological T and N stages were confirmed by surgery. Baseline CT signs of tumor length, tumor thickness, outer membrane features, total number of lymph node (tLN), short diameter of the largest lymph node (SDL), and clinical T and N stages were measured. Eight-year overall survival (OS) and disease-free survival (DFS) were estimated using Kaplan-Meier and Cox proportional hazards regression analyses to determine associations between baseline CT signs and survival outcomes. Kaplan-Meier analysis showed that tLN number, largest LN short axis diameter, pT, and pN stages all correlated with OS significantly. And the total tLN number, SDL and pN stages significantly correlated with DFS. In Cox analyses, total tLN number (>6) and pN stage were significantly associated with OS (hazard ratio [HR]: 1.55 [95% CI, 1.13-2.11, P = 0.006] and HR: 1.49 [95% CI, 1.17-1.90, P = 0.001], respectively). Cox regression analysis showed that OS index was predictive of 1- to 3-year survival. Total number of lymph node in baseline CT provides equal efficiency compared to pN stages in the prediction of 8-year long-term survival outcomes for advanced esophageal squamous cell carcinoma patients with preoperative chemotherapy.
Kanekiyo S, Takeda S, Nakajima M, et al.
Efficacy and Safety of Biweekly Docetaxel in Combination with Nedaplatin as Second-line Chemotherapy for Unresectable or Recurrent Esophageal Cancer.
Anticancer Res. 2016; 36(4):1923-7 [PubMed] Related Publications
Efficacy and Safety of Biweekly Docetaxel in Combination with Nedaplatin as Second-line Chemotherapy for Unresectable or Recurrent Esophageal Cancer.
Anticancer Res. 2016; 36(4):1923-7 [PubMed] Related Publications
AIM: In Japan, chemotherapeutic agents that have been approved for the treatment of esophageal cancer include cisplatin, nedaplatin, 5-fluorouracil, vindesine, and docetaxel. The aim of this study was to retrospectively investigate the efficacy and safety of docetaxel and nedaplatin combination chemotherapy for unresectable or recurrent esophageal cancer in an outpatient setting.
PATIENTS AND METHODS: In total, 33 patients with recurrent esophageal cancer after initial treatment (esophagectomy, chemotherapy, or chemoradiotherapy) were enrolled. Patients received docetaxel (30 mg/m(2)intravenously) and nedaplatin (30 mg/m(2)intravenously) on day 1 biweekly. The response rate (RR), time to treatment failure (TTF), overall survival time (OS), and toxicity were analyzed.
RESULTS: The median number of cycles of combination therapy was five (range=2-25 cycles). The RR was 21.2%, and the disease control rate was 60.6%. The median TTF was 71 days, and median OS was 211 days. The most frequent toxicities were leukopenia and anemia; non-hematological toxicities were generally mild. There were no treatment-related deaths.
CONCLUSION: This outpatient combination chemotherapy was useful as second-line chemotherapy for unresectable or recurrent esophageal cancer.
PATIENTS AND METHODS: In total, 33 patients with recurrent esophageal cancer after initial treatment (esophagectomy, chemotherapy, or chemoradiotherapy) were enrolled. Patients received docetaxel (30 mg/m(2)intravenously) and nedaplatin (30 mg/m(2)intravenously) on day 1 biweekly. The response rate (RR), time to treatment failure (TTF), overall survival time (OS), and toxicity were analyzed.
RESULTS: The median number of cycles of combination therapy was five (range=2-25 cycles). The RR was 21.2%, and the disease control rate was 60.6%. The median TTF was 71 days, and median OS was 211 days. The most frequent toxicities were leukopenia and anemia; non-hematological toxicities were generally mild. There were no treatment-related deaths.
CONCLUSION: This outpatient combination chemotherapy was useful as second-line chemotherapy for unresectable or recurrent esophageal cancer.
Tanaka Y, Yoshida K, Tanahashi T, et al.
Phase II trial of neoadjuvant chemotherapy with docetaxel, nedaplatin, and S1 for advanced esophageal squamous cell carcinoma.
Cancer Sci. 2016; 107(6):764-72 [PubMed] Free Access to Full Article Related Publications
Phase II trial of neoadjuvant chemotherapy with docetaxel, nedaplatin, and S1 for advanced esophageal squamous cell carcinoma.
Cancer Sci. 2016; 107(6):764-72 [PubMed] Free Access to Full Article Related Publications
Although standard chemotherapy for esophageal cancer patients is fluorouracil and cisplatin, the prognosis is still unsatisfactory. A new therapeutic regimen combining docetaxel, cisplatin, and 5-fluorouracil was recently developed to improve both local and distant tumor control. We developed a new regimen of docetaxel, nedaplatin, and S1 (DGS) and previously reported the recommended dose in a phase I dose-escalation study. We then undertook a phase II study of DGS for advanced esophageal squamous cell carcinoma. Patients with clinical stage IB/II/III disease were eligible. Patients received two courses of chemotherapy: docetaxel 35 mg/m(2) with nedaplatin 40 mg/m(2) on day 8, 80 mg/m(2) S1 on days 1-14, and 2 weeks off. After completion of chemotherapy, patients underwent esophagectomy. The primary endpoint was the completion rate of protocol treatment (completion of two courses of preoperative chemotherapy and R0 surgery [no residual tumor]). We enrolled 32 patients. The completion rate of protocol treatment was 96.9%. During chemotherapy, the most common grade 3 or 4 toxicity was neutropenia (25.0%). No treatment-related deaths were observed, and the incidence of operative morbidity was tolerable. The overall response rate after chemotherapy was 83.3%. This DGS regimen was well tolerated and highly active. This trial is registered with the University Hospital Medical Information Network (UMIN ID: 000014626).
Pang H, Feng T, Lu H, et al.
Efficacy and Safety of Nedaplatin in Advanced Breast Cancer Therapy.
Cancer Invest. 2016; 34(4):167-72 [PubMed] Related Publications
Efficacy and Safety of Nedaplatin in Advanced Breast Cancer Therapy.
Cancer Invest. 2016; 34(4):167-72 [PubMed] Related Publications
PURPOSE: To compare the time-to-treatment failure (TTF), overall survival (OS), overall response rate (ORR), and adverse effects of regimens including nedaplatin- or cisplatin-based chemotherapy for advanced breast cancer (ABC).
METHODS: A total of 171 patients with ABC (admission between July 2008 and July 2013) were retrospectively analyzed. Patients received either nedaplatin 75 mg/m(2) (arm N; n = 85) or cisplatin 75 mg/m(2) (arm C; n = 86) in combination with other second-generation chemotherapeutic drugs, such as paclitaxel 175 mg/m(2), docetaxel 75 mg/m(2), gemcitabine 1.25 g/m(2), and navelbine 25 mg/m(2) every 21 days (nedaplatin, cisplatin, paclitaxel, docetaxel on day 1; gemcitabine, navelbine on days 1 and 8). The primary endpoint was TTF in each arm; secondary endpoints were OS, ORR, and toxicity.
RESULTS: In the assessable patient population, in arm N, median TTF and OS was 13.87 months (95% CI: 11.55-16.19) and 31.53 months (95% CI: 28.42-34.64), respectively, with an ORR of 48.2%. In arm C, median TTF and OS was 8.7 months (95% CI: 5.82-11.59) and 24.87 months (95% CI: 18.98-30.75), respectively, with an ORR of 37.2%. The occurrence of grades 3 and 4 hematologic toxicity was more frequent (45.9% vs. 25.6%, p = 0.003) in arm N than in arm C. However, grade ≥2 nonhematologic toxicity was less frequent in arm N than in arm C (12.9% vs. 46.5%, p = 2.05 × 10(-7)).
CONCLUSIONS: Nedaplatin-based chemotherapy regimen was well tolerated and efficiently improved patients' quality of life characterized by prolonged TTF and OS, with a marginal ORR.
METHODS: A total of 171 patients with ABC (admission between July 2008 and July 2013) were retrospectively analyzed. Patients received either nedaplatin 75 mg/m(2) (arm N; n = 85) or cisplatin 75 mg/m(2) (arm C; n = 86) in combination with other second-generation chemotherapeutic drugs, such as paclitaxel 175 mg/m(2), docetaxel 75 mg/m(2), gemcitabine 1.25 g/m(2), and navelbine 25 mg/m(2) every 21 days (nedaplatin, cisplatin, paclitaxel, docetaxel on day 1; gemcitabine, navelbine on days 1 and 8). The primary endpoint was TTF in each arm; secondary endpoints were OS, ORR, and toxicity.
RESULTS: In the assessable patient population, in arm N, median TTF and OS was 13.87 months (95% CI: 11.55-16.19) and 31.53 months (95% CI: 28.42-34.64), respectively, with an ORR of 48.2%. In arm C, median TTF and OS was 8.7 months (95% CI: 5.82-11.59) and 24.87 months (95% CI: 18.98-30.75), respectively, with an ORR of 37.2%. The occurrence of grades 3 and 4 hematologic toxicity was more frequent (45.9% vs. 25.6%, p = 0.003) in arm N than in arm C. However, grade ≥2 nonhematologic toxicity was less frequent in arm N than in arm C (12.9% vs. 46.5%, p = 2.05 × 10(-7)).
CONCLUSIONS: Nedaplatin-based chemotherapy regimen was well tolerated and efficiently improved patients' quality of life characterized by prolonged TTF and OS, with a marginal ORR.
Quan R, Huang J, Chen N, et al.
A retrospective analysis of efficacy and safety of adding bevacizumab to chemotherapy as first- and second-line therapy in advanced non-small-cell lung cancer (NSCLC).
Tumour Biol. 2016; 37(8):11479-84 [PubMed] Related Publications
A retrospective analysis of efficacy and safety of adding bevacizumab to chemotherapy as first- and second-line therapy in advanced non-small-cell lung cancer (NSCLC).
Tumour Biol. 2016; 37(8):11479-84 [PubMed] Related Publications
Several phase III clinical trials had authenticated that the addition of bevacizumab to paclitaxel plus carboplatin or gemcitabine plus cisplatin showed encouraging efficacy as first-line therapy for advanced NSCLC patients. However, the benefits of adding bevacizumab to other chemotherapy regimens in first- or second-line therapy have not been reported. To compare the clinical efficacy and safety of bevacizumab concomitant with chemotherapy regimens in patients with advanced NSCLC as first- or second-line therapy, we retrospectively reviewed the effects of adding bevacizumab to chemotherapy regimens in naive-chemotherapy and pre-chemotherapy patients with advanced non-squamous NSCLC. A total of 79 patients with advanced non-squamous NSCLC received at least two cycles of bevacizumab with chemotherapy between October 2010 and December 2013 were selected. Our primary end points were overall response rate (ORR) and disease control rate (DCR). The secondary objective was overall survival (OS) and safety. Seventy-nine patients were included in this study. Overall response rates at first evaluation (after 2 cycles) were 23.1 % (9/39) and 5.0 % (2/40) in first- and second-line therapy (P = 0.020), respectively. And disease control rates were 84.6 % (33/39) and 50 % (20/40), respectively (P = 0.001). The median OS were 27.2 months (95 % CI 13.3-41.1 months) and 29.6 months (95 % CI 6.7-52.5 months), respectively (P = 0.740). Grade 3-4 adverse events included leukopenia (2/39), and neutropenia (3/39) in first-line therapy versus neutropenia (1/40) and thrombocytopenia (2/40) in second-line treatment. In our experience, combination of bevacizumab and chemotherapy had encouraging anti-tumor efficacy as both first- and second-line therapy.
Sakai M, Sohda M, Miyazaki T, et al.
Usefulness of 18f-Fluorodeoxyglucose Positron Emission Tomography for Predicting the Pathological Response of Neoadjuvant Chemoradiotherapy for T4 Esophageal Squamous Cell Carcinoma.
Hepatogastroenterology. 2015; 62(140):898-901 [PubMed] Related Publications
Usefulness of 18f-Fluorodeoxyglucose Positron Emission Tomography for Predicting the Pathological Response of Neoadjuvant Chemoradiotherapy for T4 Esophageal Squamous Cell Carcinoma.
Hepatogastroenterology. 2015; 62(140):898-901 [PubMed] Related Publications
BACKGROUND/AIMS: The purpose of this study is to assess the efficacy of 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) in predicting the pathological response of neoadjuvant chemoradiation (CRT) for clinically diagnosed T4 esophageal squamous cell carcinoma (SCC).
METHODOLOGY: We examined 32 patients with T4 thoracic esophageal SCC who received neoadjuvant CRT followed by surgery.
RESULTS: Pathological complete response (pCR) was achieved in 7 patients (21.9%). pCR was significant correlated with standardized uptake value (SUV) after neoadjuvant CRT (P = 0.034) and SUV decrease (%) (P = 0.030). The optimal cut-off values to predict pCR were 2.25 for SUV after neoadjuvant CRT and 79.3 for SUV decrease (%). Of note, no patients who did not reach both cut-off values achieved pCR. Conclusions: SUV after CRT and SUV decrease (%) in FDG-PET of the primary tumor are useful tools to predict pathological response of neoadjuvant CRT for T4 esophageal SCC.
METHODOLOGY: We examined 32 patients with T4 thoracic esophageal SCC who received neoadjuvant CRT followed by surgery.
RESULTS: Pathological complete response (pCR) was achieved in 7 patients (21.9%). pCR was significant correlated with standardized uptake value (SUV) after neoadjuvant CRT (P = 0.034) and SUV decrease (%) (P = 0.030). The optimal cut-off values to predict pCR were 2.25 for SUV after neoadjuvant CRT and 79.3 for SUV decrease (%). Of note, no patients who did not reach both cut-off values achieved pCR. Conclusions: SUV after CRT and SUV decrease (%) in FDG-PET of the primary tumor are useful tools to predict pathological response of neoadjuvant CRT for T4 esophageal SCC.
Dilruba S, Kalayda GV
Platinum-based drugs: past, present and future.
Cancer Chemother Pharmacol. 2016; 77(6):1103-24 [PubMed] Related Publications
Platinum-based drugs: past, present and future.
Cancer Chemother Pharmacol. 2016; 77(6):1103-24 [PubMed] Related Publications
Platinum-based drugs cisplatin, carboplatin and oxaliplatin are widely used in the therapy of human neoplasms. Their clinical success is, however, limited due to severe side effects and intrinsic or acquired resistance to the treatment. Much effort has been put into the development of new platinum anticancer complexes, but none of them has reached worldwide clinical application so far. Nedaplatin, lobaplatin and heptaplatin received only regional approval. Some new platinum complexes and platinum drug formulations are undergoing clinical trials. Here, we review the main classes of new platinum drug candidates, such as sterically hindered complexes, monofunctional platinum drugs, complexes with biologically active ligands, trans-configured and polynuclear platinum complexes, platinum(IV) prodrugs and platinum-based drug delivery systems. For each class of compounds, a detailed overview of the mechanism of action is given, the cytotoxicity is compared to that of the clinically used platinum drugs, and the clinical perspectives are discussed. A critical analysis of lessons to be learned is presented. Finally, a general outlook regarding future directions in the field of new platinum drugs is given.
Liang Z, Wang S, Lin Z, et al.
Phase I trial of nedaplatin chemotherapy concurrent with radiotherapy for untreated locoregionally advanced nasopharyngeal carcinoma.
Cancer Chemother Pharmacol. 2016; 77(3):643-51 [PubMed] Related Publications
Phase I trial of nedaplatin chemotherapy concurrent with radiotherapy for untreated locoregionally advanced nasopharyngeal carcinoma.
Cancer Chemother Pharmacol. 2016; 77(3):643-51 [PubMed] Related Publications
PURPOSE: In this phase I study, single-agent chemotherapy was conducted in patients with locoregionally advanced nasopharyngeal carcinoma (NPC) using nedaplatin (NDP) and concomitant radiotherapy. The study sought to determine the maximum tolerated dose (MTD), recommended dose (RD) and the clinical efficacy of this treatment in locoregionally advanced NPC patients.
EXPERIMENTAL DESIGN: Twenty patients were enrolled. The eligibility criteria included local advanced NPC (stage III or IVa) without any previous treatment and an expected survival of ≥ 3 months. The regimen consisted of 1.8-2.0 Gy daily radiation for 5 days a week and NDP with dose escalation of 70-100 mg/m(2) on day 1. The cycles were repeated every 21 days (day 1, day 22 and day 43) until the completion of chemoradiation. Dose-limiting toxicities (DLTs) included: grade 4 anemia; grade 4 neutropenia lasting for ≥ 5 days; grades 3 and 4 febrile neutropenia; grade 4 thrombocytopenia; grades 3-4 non-hematological toxicity (with the exception of alopecia, nausea) and any of the grade 5 responses.
RESULTS: Ninety-five percent of the assessed patients experienced a treatment response. The median time to progression among all patients was 41.9 months. Two-year overall survival was 95%, while the progression-free survival was 85%. DLT included febrile neutropenia of grade 3. The RD of NDP was 90 mg/m(2) during cycles 1-3.
CONCLUSION: NDP combined with radiotherapy and administered every 21 days for three cycles was active in patients with locoregionally advanced NPC. The regimen resulted in mild adverse effects and good patient compliance. Based on the findings from the study, the RD of NDP for phase II trial was found to be 90 mg/m(2).
EXPERIMENTAL DESIGN: Twenty patients were enrolled. The eligibility criteria included local advanced NPC (stage III or IVa) without any previous treatment and an expected survival of ≥ 3 months. The regimen consisted of 1.8-2.0 Gy daily radiation for 5 days a week and NDP with dose escalation of 70-100 mg/m(2) on day 1. The cycles were repeated every 21 days (day 1, day 22 and day 43) until the completion of chemoradiation. Dose-limiting toxicities (DLTs) included: grade 4 anemia; grade 4 neutropenia lasting for ≥ 5 days; grades 3 and 4 febrile neutropenia; grade 4 thrombocytopenia; grades 3-4 non-hematological toxicity (with the exception of alopecia, nausea) and any of the grade 5 responses.
RESULTS: Ninety-five percent of the assessed patients experienced a treatment response. The median time to progression among all patients was 41.9 months. Two-year overall survival was 95%, while the progression-free survival was 85%. DLT included febrile neutropenia of grade 3. The RD of NDP was 90 mg/m(2) during cycles 1-3.
CONCLUSION: NDP combined with radiotherapy and administered every 21 days for three cycles was active in patients with locoregionally advanced NPC. The regimen resulted in mild adverse effects and good patient compliance. Based on the findings from the study, the RD of NDP for phase II trial was found to be 90 mg/m(2).
Abou-Taleb HA, Koshiyama M, Matsumura N, et al.
Clinical efficacy of neoadjuvant chemotherapy with irinotecan (CPT-11) and nedaplatin followed by radical hysterectomy for locally advanced cervical cancer.
J Int Med Res. 2016; 44(2):346-56 [PubMed] Related Publications
Clinical efficacy of neoadjuvant chemotherapy with irinotecan (CPT-11) and nedaplatin followed by radical hysterectomy for locally advanced cervical cancer.
J Int Med Res. 2016; 44(2):346-56 [PubMed] Related Publications
OBJECTIVE: To investigate the clinical efficacy of neoadjuvant chemotherapy (NAC) with irinotecan (CPT-11) and nedaplatin (NED) followed by radical hysterectomy.
METHODS: Patients with locally advanced cervical cancer (stage Ib2-IIb) were treated with NAC followed by surgery, primary surgery or primary radiotherapy. NAC was usually performed using transuterine arterial chemotherapy (TUAC) or intravenous CPT-11/NED. Survival rates were analysed in the three treatment groups; response rates and adverse events associated with NAC, TUAC and CPT-11/NED were compared, along with previously reported adverse events of chemoradiotherapy.
RESULTS: A total of 165 patients with cervical cancer were recruited. Of these, 70 were treated with NAC followed by surgery (48 with CPT-11/NED, 18 with TUAC and four with other types of chemotherapy), 73 were treated with primary surgery and 22 with primary radiotherapy (including chemoradiotherapy). There were no significant differences in progression-free survival or overall survival rates between the three treatment groups. The response rates for the NAC regimen of CPT-11/NED and TUAC were high (75% and 78%, respectively). The frequency of severe thrombocytopenia was lower in patients receiving CPT-11/NED compared with TUAC, and the incidence of severe anaemia, vomiting and cystitis was lower in patients receiving CPT-11/NED compared with chemoradiotherapy.
CONCLUSIONS: The use of CPT-11/NED as a NAC regimen shows favourable activity, with lower toxicity compared with NAC using TUAC or chemoradiotherapy, for the treatment of locally advanced cervical cancer.
METHODS: Patients with locally advanced cervical cancer (stage Ib2-IIb) were treated with NAC followed by surgery, primary surgery or primary radiotherapy. NAC was usually performed using transuterine arterial chemotherapy (TUAC) or intravenous CPT-11/NED. Survival rates were analysed in the three treatment groups; response rates and adverse events associated with NAC, TUAC and CPT-11/NED were compared, along with previously reported adverse events of chemoradiotherapy.
RESULTS: A total of 165 patients with cervical cancer were recruited. Of these, 70 were treated with NAC followed by surgery (48 with CPT-11/NED, 18 with TUAC and four with other types of chemotherapy), 73 were treated with primary surgery and 22 with primary radiotherapy (including chemoradiotherapy). There were no significant differences in progression-free survival or overall survival rates between the three treatment groups. The response rates for the NAC regimen of CPT-11/NED and TUAC were high (75% and 78%, respectively). The frequency of severe thrombocytopenia was lower in patients receiving CPT-11/NED compared with TUAC, and the incidence of severe anaemia, vomiting and cystitis was lower in patients receiving CPT-11/NED compared with chemoradiotherapy.
CONCLUSIONS: The use of CPT-11/NED as a NAC regimen shows favourable activity, with lower toxicity compared with NAC using TUAC or chemoradiotherapy, for the treatment of locally advanced cervical cancer.
Takada A, Nakamura T, Takayama K, et al.
Preliminary treatment results of proton beam therapy with chemoradiotherapy for stage I-III esophageal cancer.
Cancer Med. 2016; 5(3):506-15 [PubMed] Free Access to Full Article Related Publications
Preliminary treatment results of proton beam therapy with chemoradiotherapy for stage I-III esophageal cancer.
Cancer Med. 2016; 5(3):506-15 [PubMed] Free Access to Full Article Related Publications
The effect of proton beam therapy (PBT) on various cancers is controversial. We aimed to evaluate the efficacy and safety of PBT with alternating chemoradiotherapy (ACRT) for patients with stage I-III esophageal cancer. Two cycles of systemic chemotherapy with a continuous infusion of 5-fluorouracil (5-FU) on days 1-5 and a 5h infusion of nedaplatin (NDP) on day 6 were accompanied by thoracic irradiation using X-ray therapy and PBT. During the first half of the treatment, X-rays were delivered to the prophylactic area. During the second half of the treatment, proton beams were used to irradiate the involved field. To reduce the dose of cardiac irradiation, proton beams were delivered with posterior and posterior oblique angles. Between January 2009 and December 2012, 47 patients were enrolled in this study. The median follow-up duration was 29 months for all patients and 40 months for survivors. The 3 year overall survival rate, progression-free survival rate, and local control rate were 59.2%, 56.3%, and 69.8%, respectively. With respect to grade 3-4 late toxicities, there were no pleural or pericardial effusions, but two patients (4.3%) had esophageal stenosis, one patient (2.1%) had fistula, and two patients (4.3%) developed radiation pneumonitis. PBT with ACRT might have the potential to reduce the risk of cardiac damage and might become one of the primary methods of esophageal cancer treatment.
Kagabu M, Shoji T, Murakami K, et al.
Clinical efficacy of nedaplatin-based concurrent chemoradiotherapy for uterine cervical cancer: a Tohoku Gynecologic Cancer Unit Study.
Int J Clin Oncol. 2016; 21(4):735-40 [PubMed] Related Publications
Clinical efficacy of nedaplatin-based concurrent chemoradiotherapy for uterine cervical cancer: a Tohoku Gynecologic Cancer Unit Study.
Int J Clin Oncol. 2016; 21(4):735-40 [PubMed] Related Publications
OBJECTIVE: The aim of this study was to compare the efficacy of nedaplatin-based concurrent chemoradiotherapy (CCRT) with that of cisplatin-based CCRT in patients with cervical cancer.
METHODS: The medical records of patients with cervical cancer who had undergone CCRT between 2003 and 2007 were retrospectively reviewed. Of these, 129 patients were treated postoperatively with CCRT (n = 52) or primary CCRT (n = 77). A total of 29 patients were treated with nedaplatin-based postoperative CCRT and 23 patients were treated with cisplatin-based postoperative CCRT. A total of 28 patients were treated with nedaplatin-based postoperative CCRT, and 49 patients were treated with cisplatin-based postoperative CCRT. Progression-free survival (PFS) and overall survival (OS) were compared between the treatment groups.
RESULTS: With postoperative CCRT, there were no significant differences in recurrence rate (P = 1.0000), PFS (log-rank: P = 0.8503), and OS (log-rank: P = 0.8926) between the two treatment groups. With primary CCRT, there were no significant differences in PFS (log-rank: P = 0.7845) and OS (log-rank: P = 0.3659). The frequency of acute toxicity was not significantly different between the cisplatin-based postoperative CCRT group and the nedaplatin-based postoperative CCRT group.
CONCLUSIONS: Nedaplatin-based postoperative CCRT is an effective and well-tolerated regimen for both early-stage and advanced-stage cervical cancer patients.
METHODS: The medical records of patients with cervical cancer who had undergone CCRT between 2003 and 2007 were retrospectively reviewed. Of these, 129 patients were treated postoperatively with CCRT (n = 52) or primary CCRT (n = 77). A total of 29 patients were treated with nedaplatin-based postoperative CCRT and 23 patients were treated with cisplatin-based postoperative CCRT. A total of 28 patients were treated with nedaplatin-based postoperative CCRT, and 49 patients were treated with cisplatin-based postoperative CCRT. Progression-free survival (PFS) and overall survival (OS) were compared between the treatment groups.
RESULTS: With postoperative CCRT, there were no significant differences in recurrence rate (P = 1.0000), PFS (log-rank: P = 0.8503), and OS (log-rank: P = 0.8926) between the two treatment groups. With primary CCRT, there were no significant differences in PFS (log-rank: P = 0.7845) and OS (log-rank: P = 0.3659). The frequency of acute toxicity was not significantly different between the cisplatin-based postoperative CCRT group and the nedaplatin-based postoperative CCRT group.
CONCLUSIONS: Nedaplatin-based postoperative CCRT is an effective and well-tolerated regimen for both early-stage and advanced-stage cervical cancer patients.
Yamashita H, Haga A, Takenaka R, et al.
Efficacy and feasibility of ambulatory treatment-based monthly nedaplatin plus S-1 in definitive or salvage concurrent chemoradiotherapy for early, advanced, and relapsed esophageal cancer.
Radiat Oncol. 2016; 11:4 [PubMed] Free Access to Full Article Related Publications
Efficacy and feasibility of ambulatory treatment-based monthly nedaplatin plus S-1 in definitive or salvage concurrent chemoradiotherapy for early, advanced, and relapsed esophageal cancer.
Radiat Oncol. 2016; 11:4 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Standard chemoradiotherapy (CRT) using cisplatin (CDDP) and 5-fluorouracil (5-FU) is an optional treatment for patients with stage II-III esophageal cancer. However, there are some demerits in this regimen because CDDP administration requires a large transfusion volume and 5-FU must be continuously infused over 24 h. Therefore, hospitalization is unavoidable. We collected retrospectively the data of definitive CRT with nedaplatin and S-1 as carried out in our institution.
METHODS: Patients with early and advanced esophageal cancer and relapsed esophageal cancer after radical surgery were included. Nedaplatin 80 mg/m(2) was given on days 1 and 29, and S-1 80 mg/m(2) on days 1-14 and 29-42. No prophylactic treatment with granulocyte colony stimulating factor was administered. Patients received two courses of concurrent radiotherapy of more than 50 Gy with or without two additional courses as adjuvant therapy every 4 weeks.
RESULTS: Between August 2011 and June 2015, 89 patients (age range, 44-86 years; K-PS 90-100, 81 %; squamous cell carcinoma histology, 97 %; definitive/salvage CRT, 75/25 %) were collected. Twenty-one (24 %) patients completed four cycles, and 94 % received two or more cycles. Grade 4 leukopenia, thrombocytopenia, and anemia occurred in 12, 7, and 10 % of the patients, respectively. Five patients developed febrile neutropenia. Grade 3 non-hematological toxicity included infection in 12 %, mucositis/esophagitis in 3 %, kidney in 3 %, and fatigue in 3 %. Sixty-four patients (72 %) received the prescribed full dose and full cycles of chemotherapy. A complete response was achieved in 76 patients (85 %). The 3-year overall survival rate was 54.4 % in definitive CRT and 39.8 % in salvage CRT, respectively. Sixty-two subjects (70 %) received treatment as outpatients.
CONCLUSIONS: Nedaplatin and S-1 in combination with radiotherapy is feasible, and toxicity is tolerable. This treatment method has the potential to shorten hospitalization without impairing the efficacy of CRT.
METHODS: Patients with early and advanced esophageal cancer and relapsed esophageal cancer after radical surgery were included. Nedaplatin 80 mg/m(2) was given on days 1 and 29, and S-1 80 mg/m(2) on days 1-14 and 29-42. No prophylactic treatment with granulocyte colony stimulating factor was administered. Patients received two courses of concurrent radiotherapy of more than 50 Gy with or without two additional courses as adjuvant therapy every 4 weeks.
RESULTS: Between August 2011 and June 2015, 89 patients (age range, 44-86 years; K-PS 90-100, 81 %; squamous cell carcinoma histology, 97 %; definitive/salvage CRT, 75/25 %) were collected. Twenty-one (24 %) patients completed four cycles, and 94 % received two or more cycles. Grade 4 leukopenia, thrombocytopenia, and anemia occurred in 12, 7, and 10 % of the patients, respectively. Five patients developed febrile neutropenia. Grade 3 non-hematological toxicity included infection in 12 %, mucositis/esophagitis in 3 %, kidney in 3 %, and fatigue in 3 %. Sixty-four patients (72 %) received the prescribed full dose and full cycles of chemotherapy. A complete response was achieved in 76 patients (85 %). The 3-year overall survival rate was 54.4 % in definitive CRT and 39.8 % in salvage CRT, respectively. Sixty-two subjects (70 %) received treatment as outpatients.
CONCLUSIONS: Nedaplatin and S-1 in combination with radiotherapy is feasible, and toxicity is tolerable. This treatment method has the potential to shorten hospitalization without impairing the efficacy of CRT.
Gridelli C, Sacco PC
Novel cytotoxic drugs in advanced nonsmall cell lung cancer.
Curr Opin Oncol. 2016; 28(2):110-4 [PubMed] Related Publications
Novel cytotoxic drugs in advanced nonsmall cell lung cancer.
Curr Opin Oncol. 2016; 28(2):110-4 [PubMed] Related Publications
PURPOSE OF REVIEW: This article focuses on novel cytotoxic drugs for the treatment of patients with advanced nonsmall cell lung cancer (NSCLC) and describes their impact on disease outcome.
RECENT FINDINGS: Nab-paclitaxel and carboplatin as first-line treatment should be considered a therapeutic option, particularly in patients with squamous histology. Nedaplatin and docetaxel improves survival in Asiatic patients with squamous histology as compared with cisplatin and docetaxel.
SUMMARY: NSCLC is a heterogeneous disease with limited available treatment options in the absence of specific molecular alterations. Defining the histological subgroup has an impact on the selection of molecular screening and therapy options. Chemotherapy has reached a plateau of effectiveness showing an overall survival of about 10 months. Therefore, some cytotoxic and antiangiogenic agents display improved efficacy in defined patient subgroups and may lead to prolonged survival. Despite this, the overall outlook of lung cancer survival for most patients remains dismal.
RECENT FINDINGS: Nab-paclitaxel and carboplatin as first-line treatment should be considered a therapeutic option, particularly in patients with squamous histology. Nedaplatin and docetaxel improves survival in Asiatic patients with squamous histology as compared with cisplatin and docetaxel.
SUMMARY: NSCLC is a heterogeneous disease with limited available treatment options in the absence of specific molecular alterations. Defining the histological subgroup has an impact on the selection of molecular screening and therapy options. Chemotherapy has reached a plateau of effectiveness showing an overall survival of about 10 months. Therefore, some cytotoxic and antiangiogenic agents display improved efficacy in defined patient subgroups and may lead to prolonged survival. Despite this, the overall outlook of lung cancer survival for most patients remains dismal.
Kimakura M, Abe T, Nagahara A, et al.
Metastatic testicular cancer presenting with liver and kidney dysfunction treated with modified BEP chemotherapy combined with continuous hemodiafiltration and rasburicase.
Anticancer Drugs. 2016; 27(4):364-8 [PubMed] Free Access to Full Article Related Publications
Metastatic testicular cancer presenting with liver and kidney dysfunction treated with modified BEP chemotherapy combined with continuous hemodiafiltration and rasburicase.
Anticancer Drugs. 2016; 27(4):364-8 [PubMed] Free Access to Full Article Related Publications
A 25-year-old man was admitted to our hospital complaining of right scrotal pain and upper abdominal pain. A computed tomographic scan indicated a right scrotal mass, a huge liver mass, and multiple lung masses, although there was no enlarged retroperitoneal lymph node swelling. Laboratory tests showed severe liver and kidney dysfunction and high levels of serum α-fetoprotein (11,997 ng/ml). Although needle biopsies of the testicular and liver masses were performed, the tissues were insufficient for a pathological diagnosis. As liver and kidney function worsened, we started chemotherapy with bleomycin, etoposide, and cisplatin (BEP chemotherapy), which was modified because of the liver and renal dysfunction. We also used continuous hemodiafiltration and rasburicase to prevent tumor lysis syndrome. After induction of chemotherapy, the liver and kidney dysfunction improved immediately and the high orchiectomy was performed on day 8 after chemotherapy. The pathological diagnosis was a yolk sac tumor. He underwent four courses of the BEP regimen and five courses of the TIN regimen (paclitaxel, ifosphamide, and nedaplatin), followed by the resection of liver metastases. There was no evidence of viable cells in the resected liver and no recurrence was evident at 1 year postoperatively.
Peng PJ, Lv BJ, Tang C, et al.
Phase II trial of docetaxel combined with nedaplatin for patients with recurrent and metastatic nasopharyngeal carcinoma.
Drug Des Devel Ther. 2015; 9:6401-5 [PubMed] Free Access to Full Article Related Publications
Phase II trial of docetaxel combined with nedaplatin for patients with recurrent and metastatic nasopharyngeal carcinoma.
Drug Des Devel Ther. 2015; 9:6401-5 [PubMed] Free Access to Full Article Related Publications
PURPOSE: This Phase II trial was designed to evaluate the efficacy and safety of docetaxel combined with nedaplatin as first-line treatment for patients with recurrent or metastatic nasopharyngeal carcinoma.
METHODS: In this multicenter Phase II trial, the patients were treated with intravenous docetaxel (75 mg/m(2), day 1) and nedaplatin (80 mg/m(2), day 1), each cycle repeated every 3 weeks for two cycles at least.
RESULTS: From January 2010 to November 2013, a total of 78 patients were recruited in this trial. Among them, 73 patients were assessable for response. The treatment was well tolerated. The main hematological adverse event was neutropenia. A total of 12 patients (15.4%) had grade 3 or grade 4 neutropenia. Grade 3 anemia was observed in six patients (7.7%) and no grade 3/4 thrombocytopenia was observed. No Grade 3/4 non-hematological toxicity was observed. There were five complete response (6.8%), 43 partial responses (58.9%), and the overall response rate was 65.8% (95% confidence interval [CI], 48.7%-81.2%). With a median follow-up period of 18.6 months, the median time to progression was 7.9 months (95% CI, 4.2-10.8 months), median overall survival was 15.7 months (95% CI, 11.6-18.5 months).
CONCLUSION: Docetaxel combined with nedaplatin offers a satisfactory clinical activity and an acceptable safety profile as first-line chemotherapy for patients with recurrent and metastatic nasopharyngeal carcinoma.
METHODS: In this multicenter Phase II trial, the patients were treated with intravenous docetaxel (75 mg/m(2), day 1) and nedaplatin (80 mg/m(2), day 1), each cycle repeated every 3 weeks for two cycles at least.
RESULTS: From January 2010 to November 2013, a total of 78 patients were recruited in this trial. Among them, 73 patients were assessable for response. The treatment was well tolerated. The main hematological adverse event was neutropenia. A total of 12 patients (15.4%) had grade 3 or grade 4 neutropenia. Grade 3 anemia was observed in six patients (7.7%) and no grade 3/4 thrombocytopenia was observed. No Grade 3/4 non-hematological toxicity was observed. There were five complete response (6.8%), 43 partial responses (58.9%), and the overall response rate was 65.8% (95% confidence interval [CI], 48.7%-81.2%). With a median follow-up period of 18.6 months, the median time to progression was 7.9 months (95% CI, 4.2-10.8 months), median overall survival was 15.7 months (95% CI, 11.6-18.5 months).
CONCLUSION: Docetaxel combined with nedaplatin offers a satisfactory clinical activity and an acceptable safety profile as first-line chemotherapy for patients with recurrent and metastatic nasopharyngeal carcinoma.
Yamada K, Saito H, Kondo T, et al.
Multicenter Phase II Study of Nedaplatin and Irinotecan for Patients with Squamous Cell Carcinoma of the Lung: Thoracic Oncology Research Group 0910.
Anticancer Res. 2015; 35(12):6705-11 [PubMed] Related Publications
Multicenter Phase II Study of Nedaplatin and Irinotecan for Patients with Squamous Cell Carcinoma of the Lung: Thoracic Oncology Research Group 0910.
Anticancer Res. 2015; 35(12):6705-11 [PubMed] Related Publications
Squamous cell carcinoma (SCC) of the lung is moderately responsive to anticancer drugs, but no specific chemotherapy regimens have yet been established. We conducted a multicenter phase II study of nedaplatin (NP) and irinotecan (CPT) for SCC of the lung. Fifty patients underwent 4 to 6 cycles of chemotherapy comprising of NP at 100 mg/m(2) on day 1 and CPT at 60 mg/m(2) on days 1 and 8 every 4 weeks. Twenty-seven patients received 4 to 6 cycles of chemotherapy (median=4 cycles). Major toxicities included neutropenia (46.0%), grade 3 or 4 anorexia (22.0%), febrile neutropenia (16.0%), diarrhea (12.0%), hyponatremia (12.0%), grade 4 anemia (10.0%), thrombocytopenia (10.0%) and infection (10.0%). There were no treatment-related deaths. One patient achieved a complete response and 16 a partial response, with an overall response rate of 34.0%. The median survival time was 11.8 months (95% CI=8.3-15.8 months) and the 2-year survival rate was 22.0%. In conclusion, the NP and CPT regimen is not recommend for further evaluation for patients with advanced SCC of the lung.
Zhang F, Zhang Y, Li WF, et al.
Efficacy of Concurrent Chemotherapy for Intermediate Risk NPC in the Intensity-Modulated Radiotherapy Era: a Propensity-Matched Analysis.
Sci Rep. 2015; 5:17378 [PubMed] Free Access to Full Article Related Publications
Efficacy of Concurrent Chemotherapy for Intermediate Risk NPC in the Intensity-Modulated Radiotherapy Era: a Propensity-Matched Analysis.
Sci Rep. 2015; 5:17378 [PubMed] Free Access to Full Article Related Publications
This study is to evaluate the efficacy of additional concurrent chemotherapy for intermediate risk (stage II and T3N0M0) NPC patients treated with intensity-modulated radiotherapy (IMRT).440 patients with intermediate risk NPC were studied retrospectively, including 128 patients treated with IMRT alone [radiotherapy group (RT group)] and 312 paitents treated with IMRT plus concurrent chemotherapy [chemoradiotherapy group (CRT group)]. Propensity score matching was carried out to create RT and CRT cohorts equally matched for host and tumor factor. Significantly more severe acute toxicities were observed in the CRT group than in the RT group. Multivariate analyses of 440 patients failed to demonstrate concurrent chemotherapy as an independent prognostic factor for FFS, LR-FFS, and D-FFS. Between the well-matched RT cohort and the CRT cohort, no significant difference was demonstrated in all survival endpoints (FFS: 92.8% versus 91.2%, P = 0.801; LR-FFS: 95.2% versus 94.4%, P = 0.755; D-FFS: 96.4% versus 96.3%, P = 0.803; OS: 98.2% versus 98.9%, P = 0.276). Our results demonstrated that for patients with intermediate risk NPC treated with IMRT, additional concurrent chemotherapy did not provide any significant survival benefit but significantly more severe acute toxicities. However, prospective randomized trials are warranted for the ultimate confirm of our findings.
Shukuya T, Yamanaka T, Seto T, et al.
Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): a randomised, open-label, phase 3 trial.
Lancet Oncol. 2015; 16(16):1630-8 [PubMed] Related Publications
Nedaplatin plus docetaxel versus cisplatin plus docetaxel for advanced or relapsed squamous cell carcinoma of the lung (WJOG5208L): a randomised, open-label, phase 3 trial.
Lancet Oncol. 2015; 16(16):1630-8 [PubMed] Related Publications
BACKGROUND: The combination of nedaplatin, a cisplatin derivative, and docetaxel showed promising activity for advanced squamous cell lung carcinoma in a previous phase 1-2 study. We compared nedaplatin plus docetaxel with cisplatin plus docetaxel in patients with previously untreated advanced or relapsed squamous cell lung carcinoma to determine effects on overall survival.
METHODS: We did a randomised, open-label, phase 3 study at 53 institutions in Japan. Eligibility criteria included pathologically proven squamous cell lung cancer with stage IIIB/IV or postoperative recurrence, age 20-74 years, Eastern Cooperative Oncology Group performance status of 0-1, no previous chemotherapy or recurrence more than a year after previous adjuvant chemotherapy, and adequate organ function. Patients were randomly assigned (1:1) to 100 mg/m(2) nedaplatin and 60 mg/m(2) docetaxel intravenously, or 80 mg/m(2) cisplatin and 60 mg/m(2) docetaxel, every 3 weeks for four to six cycles (at the treating oncologist's discretion). Randomisation was done centrally at the West Japan Oncology Group data centre via a computer-generated allocation sequence with dynamic minimisation that balanced stage (IIIB/IV or postoperative recurrent), sex, and institution. The primary endpoint was overall survival in the modified intention-to-treat population (ie, all patients who were randomly assigned and met the inclusion criteria). Safety analyses were done in all randomly assigned patients who received at least one dose of the study regimen. This trial is registered with the UMIN Clinical Trials Registry, number UMIN000002015, and is closed to new participants.
FINDINGS: Between July 6, 2009, and July 26, 2012, 355 patients were randomly assigned. 349 patients were included in the modified intention-to-treat analysis (177 in the nedaplatin group and 172 in the cisplatin group). Overall survival was significantly longer in the nedaplatin group (median 13·6 months, 95% CI 11·6-15·6) than in the cisplatin group (11·4 months,10·2-12·2; hazard ratio 0·81, 95% CI 0·65-1·02; p=0·037, one-sided stratified log-rank test). Grade 3 or worse nausea (seven of 177 patients in the nedaplatin group and 25 of 175 in the cisplatin group), fatigue (six vs 20), hyponatraemia (24 vs 53), and hypokalaemia (four vs 15) were more frequent in the cisplatin group than in the nedaplatin group, whereas grade 3 or worse leucopenia (98 vs 77), neutropenia (146 vs 123), and thrombocytopenia (16 vs none) were more frequent in the nedaplatin group than in the cisplatin group. Treatment-related deaths occurred in four and three patients in nedaplatin and cisplatin groups, respectively.
INTERPRETATION: Overall survival was significantly longer with nedaplatin plus docetaxel than with cisplatin plus docetaxel, and the regimens had different safety profiles. Nedaplatin plus docetaxel could be a new treatment option for advanced or relapsed squamous cell lung cancer.
FUNDING: West Japan Oncology Group and Sanofi.
METHODS: We did a randomised, open-label, phase 3 study at 53 institutions in Japan. Eligibility criteria included pathologically proven squamous cell lung cancer with stage IIIB/IV or postoperative recurrence, age 20-74 years, Eastern Cooperative Oncology Group performance status of 0-1, no previous chemotherapy or recurrence more than a year after previous adjuvant chemotherapy, and adequate organ function. Patients were randomly assigned (1:1) to 100 mg/m(2) nedaplatin and 60 mg/m(2) docetaxel intravenously, or 80 mg/m(2) cisplatin and 60 mg/m(2) docetaxel, every 3 weeks for four to six cycles (at the treating oncologist's discretion). Randomisation was done centrally at the West Japan Oncology Group data centre via a computer-generated allocation sequence with dynamic minimisation that balanced stage (IIIB/IV or postoperative recurrent), sex, and institution. The primary endpoint was overall survival in the modified intention-to-treat population (ie, all patients who were randomly assigned and met the inclusion criteria). Safety analyses were done in all randomly assigned patients who received at least one dose of the study regimen. This trial is registered with the UMIN Clinical Trials Registry, number UMIN000002015, and is closed to new participants.
FINDINGS: Between July 6, 2009, and July 26, 2012, 355 patients were randomly assigned. 349 patients were included in the modified intention-to-treat analysis (177 in the nedaplatin group and 172 in the cisplatin group). Overall survival was significantly longer in the nedaplatin group (median 13·6 months, 95% CI 11·6-15·6) than in the cisplatin group (11·4 months,10·2-12·2; hazard ratio 0·81, 95% CI 0·65-1·02; p=0·037, one-sided stratified log-rank test). Grade 3 or worse nausea (seven of 177 patients in the nedaplatin group and 25 of 175 in the cisplatin group), fatigue (six vs 20), hyponatraemia (24 vs 53), and hypokalaemia (four vs 15) were more frequent in the cisplatin group than in the nedaplatin group, whereas grade 3 or worse leucopenia (98 vs 77), neutropenia (146 vs 123), and thrombocytopenia (16 vs none) were more frequent in the nedaplatin group than in the cisplatin group. Treatment-related deaths occurred in four and three patients in nedaplatin and cisplatin groups, respectively.
INTERPRETATION: Overall survival was significantly longer with nedaplatin plus docetaxel than with cisplatin plus docetaxel, and the regimens had different safety profiles. Nedaplatin plus docetaxel could be a new treatment option for advanced or relapsed squamous cell lung cancer.
FUNDING: West Japan Oncology Group and Sanofi.
Umezawa R, Jingu K, Matsushita H, et al.
Long-term results of chemoradiotherapy for stage II-III thoracic esophageal cancer in a single institution after 2000 -with a focus on comparison of three protocols.
BMC Cancer. 2015; 15:813 [PubMed] Free Access to Full Article Related Publications
Long-term results of chemoradiotherapy for stage II-III thoracic esophageal cancer in a single institution after 2000 -with a focus on comparison of three protocols.
BMC Cancer. 2015; 15:813 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: To evaluate the long-term results of chemoradiotherapy (CRT) for stage II-III thoracic esophageal cancer mainly by comparing results of three protocols retrospectively.
METHODS: Between 2000 and 2012, 298 patients with stage II-III thoracic esophageal cancer underwent CRT. Patients in Group A received two cycles of cisplatin (CDDP) at 70 mg/m(2) (day 1 and 29) and 5-fluorouracil (5-FU) at 700 mg/m(2)/24 h (day 1-4 and 29-32) with radiotherapy (RT) of 60 Gy without a break. Patients in Group B received two cycles of CDDP at 40 mg/m(2) (day 1, 8, 36 and 43) and 5-FU at 400 mg/m(2)/24 h (day 1-5, 8-12, 36-40 and 43-47) with RT of 60 Gy with a 2-week break. Patients in Group C received two cycles of nedaplatin at 70 mg/m(2) (day 1 and 29) and 5-FU at 500 mg/m(2)/24 h (day 1-4 and 29-32) with RT of 60-70 Gy without a break. Differences in prognostic factors between the groups were analyzed by univariate and multivariate analyses.
RESULTS: The 5-year overall survival rates for patients in Group A, Group B and Group C were 52.4, 45.2 and 37.2%, respectively. The 5-year overall survival rates for patients in Stage II, Stage III (non-T4) and Stage III (T4) were 64.0, 40.1 and 22.5%, respectively. The 5-year overall survival rates for patients who received 1 cycle and 2 cycles of concomitant chemotherapy were 27.9 and 46.0%, respectively. In univariate analysis, stage, performance status and number of concomitant chemotherapy cycles were significant prognostic factors (p < 0.001, p = 0.008 and p < 0.001, respectively). In multivariate analysis, stage, protocol and number of concomitant chemotherapy cycles were significant factors (p < 0.001, p = 0.043 and p < 0.001, respectively).
CONCLUSIONS: The protocol used in Group A may be an effective protocol of CRT for esophageal cancer. It may be important to complete the scheduled concomitant chemotherapy with the appropriate intensity of CRT.
METHODS: Between 2000 and 2012, 298 patients with stage II-III thoracic esophageal cancer underwent CRT. Patients in Group A received two cycles of cisplatin (CDDP) at 70 mg/m(2) (day 1 and 29) and 5-fluorouracil (5-FU) at 700 mg/m(2)/24 h (day 1-4 and 29-32) with radiotherapy (RT) of 60 Gy without a break. Patients in Group B received two cycles of CDDP at 40 mg/m(2) (day 1, 8, 36 and 43) and 5-FU at 400 mg/m(2)/24 h (day 1-5, 8-12, 36-40 and 43-47) with RT of 60 Gy with a 2-week break. Patients in Group C received two cycles of nedaplatin at 70 mg/m(2) (day 1 and 29) and 5-FU at 500 mg/m(2)/24 h (day 1-4 and 29-32) with RT of 60-70 Gy without a break. Differences in prognostic factors between the groups were analyzed by univariate and multivariate analyses.
RESULTS: The 5-year overall survival rates for patients in Group A, Group B and Group C were 52.4, 45.2 and 37.2%, respectively. The 5-year overall survival rates for patients in Stage II, Stage III (non-T4) and Stage III (T4) were 64.0, 40.1 and 22.5%, respectively. The 5-year overall survival rates for patients who received 1 cycle and 2 cycles of concomitant chemotherapy were 27.9 and 46.0%, respectively. In univariate analysis, stage, performance status and number of concomitant chemotherapy cycles were significant prognostic factors (p < 0.001, p = 0.008 and p < 0.001, respectively). In multivariate analysis, stage, protocol and number of concomitant chemotherapy cycles were significant factors (p < 0.001, p = 0.043 and p < 0.001, respectively).
CONCLUSIONS: The protocol used in Group A may be an effective protocol of CRT for esophageal cancer. It may be important to complete the scheduled concomitant chemotherapy with the appropriate intensity of CRT.
Yin L, Bian XH, Wang X, et al.
Long-Term Results of Concurrent Chemoradiotherapy for Advanced N2-3 Stage Nasopharyngeal Carcinoma.
PLoS One. 2015; 10(9):e0137383 [PubMed] Free Access to Full Article Related Publications
Long-Term Results of Concurrent Chemoradiotherapy for Advanced N2-3 Stage Nasopharyngeal Carcinoma.
PLoS One. 2015; 10(9):e0137383 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: N-stage is related to distant metastasis in nasopharyngeal carcinoma (NPC) patients. The purpose of this study was to evaluate the efficacy and toxicity of different nedaplatin-based chemotherapy regimens in advanced N2-3 stage NPC patients treated with intensity modulated radiation therapy (IMRT).
PATIENTS AND METHODS: Between April 2005 and December 2009, a total of 128 patients with N2-3 advanced NPC were retrospectively analyzed. Patients were treated with IMRT concurrent with 2 cycles of chemotherapy consisting of either nedaplatin plus paclitaxel (NP group, n = 67) or nedaplatin plus fluorouracil and paclitaxel (NFP group, n = 61). Two to four cycles of adjuvant chemotherapy were then administered every 21 days following concurrent chemoradiotherapy.
RESULTS: With a median follow-up of 60 months, the 5-year overall survival (OS), progression-free survival (PFS), local-regional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS) for all patients were 81.4%, 71.5%, 87.8% and 82.0%, respectively. No significant difference in PFS (66.6% vs. 76.7%, P = 0.212) and LRRFS rates (89.0% vs. 86.3%, P = 0.664) was observed between the NP and NFP groups. The 5-year OS (75.4% vs. 88.5%, P = 0.046) and DMFS (75.1% vs. 89.0%, P = 0.042) rate were superior in the NFP group compared with the NP group. The NFP group had a higher incidence of grade 3-4 acute toxicities including bone marrow suppression (leukopenia: χ2 = 3.935, P = 0.047; anemia: χ2 = 9.760, P = 0.002; thrombocytopenia: χ2 = 8.821, P = 0.003), and both liver and renal dysfunction (χ2 = 5.206, P = 0.023) compared with the NP group. Late toxicities were moderate and no difference was observed between the two groups.
CONCLUSION: IMRT concurrent with nedaplatin-based chemotherapy is an advocated regimen for patients with advanced N2-3 stage NPC. Patients with advanced N2-3 stage may be better candidates for the NFP regimen although this regimen was associated with a high acute toxicity rate.
PATIENTS AND METHODS: Between April 2005 and December 2009, a total of 128 patients with N2-3 advanced NPC were retrospectively analyzed. Patients were treated with IMRT concurrent with 2 cycles of chemotherapy consisting of either nedaplatin plus paclitaxel (NP group, n = 67) or nedaplatin plus fluorouracil and paclitaxel (NFP group, n = 61). Two to four cycles of adjuvant chemotherapy were then administered every 21 days following concurrent chemoradiotherapy.
RESULTS: With a median follow-up of 60 months, the 5-year overall survival (OS), progression-free survival (PFS), local-regional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS) for all patients were 81.4%, 71.5%, 87.8% and 82.0%, respectively. No significant difference in PFS (66.6% vs. 76.7%, P = 0.212) and LRRFS rates (89.0% vs. 86.3%, P = 0.664) was observed between the NP and NFP groups. The 5-year OS (75.4% vs. 88.5%, P = 0.046) and DMFS (75.1% vs. 89.0%, P = 0.042) rate were superior in the NFP group compared with the NP group. The NFP group had a higher incidence of grade 3-4 acute toxicities including bone marrow suppression (leukopenia: χ2 = 3.935, P = 0.047; anemia: χ2 = 9.760, P = 0.002; thrombocytopenia: χ2 = 8.821, P = 0.003), and both liver and renal dysfunction (χ2 = 5.206, P = 0.023) compared with the NP group. Late toxicities were moderate and no difference was observed between the two groups.
CONCLUSION: IMRT concurrent with nedaplatin-based chemotherapy is an advocated regimen for patients with advanced N2-3 stage NPC. Patients with advanced N2-3 stage may be better candidates for the NFP regimen although this regimen was associated with a high acute toxicity rate.
Yang S
A Pooled Study on Combination of Gemcitabine and Nedaplatin for Treating Patients with Non-small Cell Lung Cancer.
Asian Pac J Cancer Prev. 2015; 16(14):5963-6 [PubMed] Related Publications
A Pooled Study on Combination of Gemcitabine and Nedaplatin for Treating Patients with Non-small Cell Lung Cancer.
Asian Pac J Cancer Prev. 2015; 16(14):5963-6 [PubMed] Related Publications
BACKGROUND: This analysis was conducted to evaluate the efficacy and safety of a combination of gemcitabine and nedaplatin in treating patients with non-small cell lung cancer.
METHODS: Clinical studies evaluating the efficacy and safety of a combination of gemcitabine and nedaplatin with attention to response and safety for patients with non-small cell lung cancer were identified using a predefined search strategy. Pooled response rates for gemcitabine and nedaplatin were calculated.
RESULTS: In gemcitabine and nedaplatin based regimens, 4 clinical studies including 112 patients with non-small cell lung cancer were considered eligible for inclusion. The pooled analysis suggested that the pooled reponse rate was 40.2% (45/112). Main side effects included grade 3-4 neutropenia, thrombocytopenia, and anemia. Grade 3-4 nonhematological toxicity included nausea and vomiting, diarrhea, and hepatic dysfunction. There were no treatment-related deaths.
CONCLUSION: This evidence based analysis suggests that the combination of gemcitabine and nedaplatin is associated with good response rate and accepted toxicity for treating patients with non-small cell lung cancer.
METHODS: Clinical studies evaluating the efficacy and safety of a combination of gemcitabine and nedaplatin with attention to response and safety for patients with non-small cell lung cancer were identified using a predefined search strategy. Pooled response rates for gemcitabine and nedaplatin were calculated.
RESULTS: In gemcitabine and nedaplatin based regimens, 4 clinical studies including 112 patients with non-small cell lung cancer were considered eligible for inclusion. The pooled analysis suggested that the pooled reponse rate was 40.2% (45/112). Main side effects included grade 3-4 neutropenia, thrombocytopenia, and anemia. Grade 3-4 nonhematological toxicity included nausea and vomiting, diarrhea, and hepatic dysfunction. There were no treatment-related deaths.
CONCLUSION: This evidence based analysis suggests that the combination of gemcitabine and nedaplatin is associated with good response rate and accepted toxicity for treating patients with non-small cell lung cancer.
Isohashi F, Mabuchi S, Yoshioka Y, et al.
Intensity-modulated radiation therapy versus three-dimensional conformal radiation therapy with concurrent nedaplatin-based chemotherapy after radical hysterectomy for uterine cervical cancer: comparison of outcomes, complications, and dose-volume histogram parameters.
Radiat Oncol. 2015; 10:180 [PubMed] Free Access to Full Article Related Publications
Intensity-modulated radiation therapy versus three-dimensional conformal radiation therapy with concurrent nedaplatin-based chemotherapy after radical hysterectomy for uterine cervical cancer: comparison of outcomes, complications, and dose-volume histogram parameters.
Radiat Oncol. 2015; 10:180 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: The purpose of this study is to report our clinical outcomes using intensity-modulated radiation therapy (IMRT) for adjuvant treatment of cervical cancer, compared with three-dimensional conformal radiation therapy (3DCRT), in terms of tumor control, complications and dose-volume histogram (DVH) parameters.
METHODS: Between March 2008 and February 2014, 62 patients were treated with concurrent nedaplatin-based chemotherapy and whole-pelvic external beam radiation therapy (RT). Of these patients, 32 (52%) received 3DCRT and 30 (48%) received IMRT.
RESULTS: The median follow-up periods were 40 months (range 2-74 months). The 3-year overall survival rate (OS), locoregional control rate (LRC) and progression-free survival rate (PFS) were 92, 95 and 92% in the IMRT group, and 85, 82 and 70% in the 3DCRT group, respectively. A comparison of OS, LRC and PFS showed no significant differences between IMRT and 3DCRT. The 3-year cumulative incidences of grade 2 or higher chronic gastrointestinal (GI) complications were significantly lower with IMRT compared to 3DCRT (3% vs. 45%, p < .02) and in patients with V40 of the small bowel loops of ≤340 mL compared to those with >340 mL (3% vs. 45%, p < .001). Patients treated with IMRT had a higher incidence of grade 3 acute hematologic complications (p < .05). V40 and V45 of the small bowel loops or bowel bag were predictive for development of both acute and chronic GI complications.
CONCLUSIONS: Our results suggest that IMRT for adjuvant treatment of cervical cancer is useful for decreasing GI complications without worsening outcomes.
METHODS: Between March 2008 and February 2014, 62 patients were treated with concurrent nedaplatin-based chemotherapy and whole-pelvic external beam radiation therapy (RT). Of these patients, 32 (52%) received 3DCRT and 30 (48%) received IMRT.
RESULTS: The median follow-up periods were 40 months (range 2-74 months). The 3-year overall survival rate (OS), locoregional control rate (LRC) and progression-free survival rate (PFS) were 92, 95 and 92% in the IMRT group, and 85, 82 and 70% in the 3DCRT group, respectively. A comparison of OS, LRC and PFS showed no significant differences between IMRT and 3DCRT. The 3-year cumulative incidences of grade 2 or higher chronic gastrointestinal (GI) complications were significantly lower with IMRT compared to 3DCRT (3% vs. 45%, p < .02) and in patients with V40 of the small bowel loops of ≤340 mL compared to those with >340 mL (3% vs. 45%, p < .001). Patients treated with IMRT had a higher incidence of grade 3 acute hematologic complications (p < .05). V40 and V45 of the small bowel loops or bowel bag were predictive for development of both acute and chronic GI complications.
CONCLUSIONS: Our results suggest that IMRT for adjuvant treatment of cervical cancer is useful for decreasing GI complications without worsening outcomes.
Liu Z, Liu J, Li L, et al.
Inhibition of Autophagy Potentiated the Antitumor Effect of Nedaplatin in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells.
PLoS One. 2015; 10(8):e0135236 [PubMed] Free Access to Full Article Related Publications
Inhibition of Autophagy Potentiated the Antitumor Effect of Nedaplatin in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells.
PLoS One. 2015; 10(8):e0135236 [PubMed] Free Access to Full Article Related Publications
Nedaplatin, a cisplatin analog, was developed to reduce the toxicity of cisplatin, whereas it can be cross-resistant with cisplatin in some circumstances. This study aimed to investigate the role of autophagy in nedaplatin induced cell death in cisplatin-resistant nasopharyngeal carcinoma cells. Here, we showed that HNE1/DDP and CNE2/DDP cells were resistant to nedaplatin-induced cell death with reduced apoptotic activity. Nedaplatin treatment resulted in autophagosome accumulation and increased expression of LC3-II, indicating the induction of autophagy by nedaplatin in HNE1/DDP and CNE2/DDP cells. Inhibition of autophagy by Bafilomycin A1 (Baf A1) and 3-Methyladenine (3-MA) remarkably enhanced the antitumor efficacy of nedaplatin in HNE1/DDP and CNE2/DDP cells, suggesting that the resistance to nedaplatin-induced cell death was caused by enhanced autophagy in nedaplatin-resistant NPC cells. Additionally, Baf A1 enhanced reactive oxygen species (ROS) generation and apoptosis induced by nedaplatin in HNE1/DDP cells. Mechanistically, nedaplatin treatment caused activation of ERK1/2 and suppression of Akt/mTOR signaling pathways. While inhibition of ERK1/2 by MEK1/2 inhibitor, U0126, could reduce the expression of LC3-II in nedaplatin-resistant NPC cells. Furthermore, suppression of ROS could inhibit nedaplatin-induced ERK activation in HNE1/DDP cells, indicating that ROS and ERK were involved in nedaplatin-induced autophagy. Together, these findings suggested that autophagy played a cytoprotective role in nedaplatin-induced cytotoxicity of HNE1/DDP and CNE2/DDP cells. Furthermore, our results highlighted a potential approach to restore the sensitivity of cisplatin-resistant nasopharyngeal cancer cells to nedaplatin in combination with autophagy inhibitors.
Yamashita H, Takenaka R, Omori M, et al.
Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study.
Radiat Oncol. 2015; 10:171 [PubMed] Free Access to Full Article Related Publications
Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study.
Radiat Oncol. 2015; 10:171 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: This retrospective study on early and locally advanced esophageal cancer was conducted to evaluate locoregional failure and its impact on survival by comparing involved field radiotherapy (IFRT) with elective nodal irradiation (ENI) in combination with concurrent chemotherapy.
METHODS: We assessed all patients with esophageal cancer of stages I-IV treated with definitive radiotherapy from June 2000 to March 2014. Between 2000 and 2011, ENI was used for all cases excluding high age cases. After Feb 2011, a prospective study about IFRT was started, and therefore IFRT was used since then for all cases. Concurrent chemotherapy regimen was nedaplatin (80 mg/m(2) at D1 and D29) and 5-fluorouracil (800 mg/m(2) at D1-4 and D29-32).
RESULTS: Of the 239 consecutive patients assessed (120 ENI vs. 119 IFRT), 59 patients (24.7%) had stage IV disease and all patients received at least one cycle of chemotherapy. The median follow-up time for survivors was 34.0 months. There were differences in 3-year local control (44.8% vs. 55.5%, p = 0.039), distant control (53.8% vs. 69.9%, p = 0.021) and overall survival (34.8% vs. 51.6%, p = 0.087) rates between ENI vs. IFRT, respectively. Patients treated with IFRT (8 %) demonstrated a significantly lower risk (p = 0.047) of high grade late toxicities than with ENI (16%). IFRT did not increase the risk of initially uninvolved or isolated nodal failures (27.5% in ENI and 13.4% in IFRT).
CONCLUSIONS: Nodal failure rates in clinically uninvolved nodal stations were not increased with IFRT when compared to ENI. IFRT also resulted in significantly decreased esophageal toxicity, suggesting that IFRT may allow for integration of concurrent systemic chemotherapy in a greater proportion of patients. Both tendencies of improved loco-regional progression-free survival and a significant increased overall survival rate favored the IFRT arm over the ENI arm in this study.
METHODS: We assessed all patients with esophageal cancer of stages I-IV treated with definitive radiotherapy from June 2000 to March 2014. Between 2000 and 2011, ENI was used for all cases excluding high age cases. After Feb 2011, a prospective study about IFRT was started, and therefore IFRT was used since then for all cases. Concurrent chemotherapy regimen was nedaplatin (80 mg/m(2) at D1 and D29) and 5-fluorouracil (800 mg/m(2) at D1-4 and D29-32).
RESULTS: Of the 239 consecutive patients assessed (120 ENI vs. 119 IFRT), 59 patients (24.7%) had stage IV disease and all patients received at least one cycle of chemotherapy. The median follow-up time for survivors was 34.0 months. There were differences in 3-year local control (44.8% vs. 55.5%, p = 0.039), distant control (53.8% vs. 69.9%, p = 0.021) and overall survival (34.8% vs. 51.6%, p = 0.087) rates between ENI vs. IFRT, respectively. Patients treated with IFRT (8 %) demonstrated a significantly lower risk (p = 0.047) of high grade late toxicities than with ENI (16%). IFRT did not increase the risk of initially uninvolved or isolated nodal failures (27.5% in ENI and 13.4% in IFRT).
CONCLUSIONS: Nodal failure rates in clinically uninvolved nodal stations were not increased with IFRT when compared to ENI. IFRT also resulted in significantly decreased esophageal toxicity, suggesting that IFRT may allow for integration of concurrent systemic chemotherapy in a greater proportion of patients. Both tendencies of improved loco-regional progression-free survival and a significant increased overall survival rate favored the IFRT arm over the ENI arm in this study.
 Carboplatin
Carboplatin