Fluorouracil
"A pyrimidine analog that is an antineoplastic antimetabolite. It interferes with DNA synthesis by blocking the THYMIDYLATE SYNTHETASE conversion of deoxyuridylic acid to thymidylic acid." (MeSH 2013)
Found this page useful?
 Web Resources: Fluorouracil
Web Resources: Fluorouracil Latest Research Publications
Latest Research PublicationsWeb Resources: Fluorouracil (6 links)
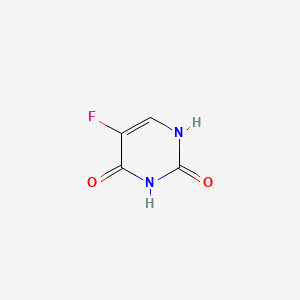
 5-Fluorouracil - Substance Summary
5-Fluorouracil - Substance Summary
PubChem
Cancer Research UK
Macmillan Cancer Support
NHS Evidence
Irish Cancer Society
MedlinePlus
Latest Research Publications
This list of publications is regularly updated (Source: PubMed).
Chen J, Wang J, Xu T
Comparison of efficacy and safety of S-1 and capecitabine in patients with metastatic colorectal carcinoma: A systematic review and meta-analysis.
Medicine (Baltimore). 2019; 98(30):e16667 [PubMed] Related Publications
Comparison of efficacy and safety of S-1 and capecitabine in patients with metastatic colorectal carcinoma: A systematic review and meta-analysis.
Medicine (Baltimore). 2019; 98(30):e16667 [PubMed] Related Publications
BACKGROUND: This study aimed to compare the efficacy and safety of S-1 and capecitabine in patients with metastatic colorectal carcinoma (mCRC).
METHODS: Eligible prospective clinical trials were searched and available data were extracted. Odds ratio and hazard ratio of available outcomes including objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and adverse events (AEs) were pooled for analysis.
RESULTS: A total of 6 studies including 828 patients were included. The results of pooled analysis showed no statistical difference in short-term efficacy including ORR (95% confidence interval [CI]: 0.68-1.19; P = .48) or DCR (95% CI: 0.65-1.29; P = .61), or long-term efficacy including PFS (95% CI: 0.75-1.08; P = .26) or OS (95% CI: 0.78-1.13; P = .50). Symptoms of diarrhea at any grade were more prevalent (95% CI: 1.21-2.29; P = .002) in patients treated with S-1, while hand-foot syndrome (HFS) at any grade (95% CI: 0.24-0.48; P < .0001) or high grade (95% CI: 0.09-0.48; P < .0001) was more frequent in capecitabine group. AEs including leucopenia, neutropenia, anemia, thrombocytopenia, vomiting, oral mucositis, stomatitis, elevated alanine transaminase, or peripheral neuropathy showed no statistical difference between S-1 and capecitabine group (all P > .05).
CONCLUSIONS: This meta-analysis reveals that S-1 has comparable efficacy, lower risk of HFS and higher incidence of diarrhea compared to capecitabine for treatment in patients with mCRC.
METHODS: Eligible prospective clinical trials were searched and available data were extracted. Odds ratio and hazard ratio of available outcomes including objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and adverse events (AEs) were pooled for analysis.
RESULTS: A total of 6 studies including 828 patients were included. The results of pooled analysis showed no statistical difference in short-term efficacy including ORR (95% confidence interval [CI]: 0.68-1.19; P = .48) or DCR (95% CI: 0.65-1.29; P = .61), or long-term efficacy including PFS (95% CI: 0.75-1.08; P = .26) or OS (95% CI: 0.78-1.13; P = .50). Symptoms of diarrhea at any grade were more prevalent (95% CI: 1.21-2.29; P = .002) in patients treated with S-1, while hand-foot syndrome (HFS) at any grade (95% CI: 0.24-0.48; P < .0001) or high grade (95% CI: 0.09-0.48; P < .0001) was more frequent in capecitabine group. AEs including leucopenia, neutropenia, anemia, thrombocytopenia, vomiting, oral mucositis, stomatitis, elevated alanine transaminase, or peripheral neuropathy showed no statistical difference between S-1 and capecitabine group (all P > .05).
CONCLUSIONS: This meta-analysis reveals that S-1 has comparable efficacy, lower risk of HFS and higher incidence of diarrhea compared to capecitabine for treatment in patients with mCRC.
Hwang JE, Kim H, Shim HJ, et al.
Lymph-node ratio is an important clinical determinant for selecting the appropriate adjuvant chemotherapy regimen for curative D2-resected gastric cancer.
J Cancer Res Clin Oncol. 2019; 145(8):2157-2166 [PubMed] Related Publications
Lymph-node ratio is an important clinical determinant for selecting the appropriate adjuvant chemotherapy regimen for curative D2-resected gastric cancer.
J Cancer Res Clin Oncol. 2019; 145(8):2157-2166 [PubMed] Related Publications
PURPOSE: Adjuvant chemotherapy for gastric cancer, particularly stage III, improves survival after curative D2 gastrectomy. We investigated the clinical value of the lymph-node ratio (LNR; number of metastatic lymph nodes/number of lymph nodes examined) for selecting the appropriate adjuvant chemotherapy regimen in patients with D2-resected stage II/III gastric cancer.
METHODS: We reviewed the data of 819 patients who underwent curative D2 gastrectomy followed by adjuvant chemotherapy. Of them, 353 patients received platinum-based chemotherapy and 466 received TS-1. The patients were categorized into three groups according to their LNR (LNR 1, 0-0.1; LNR 2, > 0.1-0.25; and LNR 3, > 0.25), and their disease-free survival (DFS) was evaluated.
RESULTS: The DFS curves of the patients were well separated according to stage and LNR. In multivariate analyses, an LNR > 0.1 was strongly associated with the 3-year DFS (hazard ratio 2.402, 95% confidence interval 1.607-3.590, P < 0.001). Platinum-based chemotherapy improved the 3-year DFS compared to TS-1 in patients with LNR 3 group in stage III gastric cancer (platinum vs. TS-1, median DFS 26.87 vs. 16.27 months, P = 0.028). An LNR > 0.1 was associated with benefiting from platinum-based adjuvant chemotherapy in stage III gastric cancer patients with lymphovascular invasion (platinum vs. TS-1, median DFS 47.57 vs. 21.77 months, P = 0.011).
CONCLUSIONS: The LNR can be used to select the appropriate adjuvant chemotherapy regimen for patients with D2-resected gastric cancer, particularly in stage III.
METHODS: We reviewed the data of 819 patients who underwent curative D2 gastrectomy followed by adjuvant chemotherapy. Of them, 353 patients received platinum-based chemotherapy and 466 received TS-1. The patients were categorized into three groups according to their LNR (LNR 1, 0-0.1; LNR 2, > 0.1-0.25; and LNR 3, > 0.25), and their disease-free survival (DFS) was evaluated.
RESULTS: The DFS curves of the patients were well separated according to stage and LNR. In multivariate analyses, an LNR > 0.1 was strongly associated with the 3-year DFS (hazard ratio 2.402, 95% confidence interval 1.607-3.590, P < 0.001). Platinum-based chemotherapy improved the 3-year DFS compared to TS-1 in patients with LNR 3 group in stage III gastric cancer (platinum vs. TS-1, median DFS 26.87 vs. 16.27 months, P = 0.028). An LNR > 0.1 was associated with benefiting from platinum-based adjuvant chemotherapy in stage III gastric cancer patients with lymphovascular invasion (platinum vs. TS-1, median DFS 47.57 vs. 21.77 months, P = 0.011).
CONCLUSIONS: The LNR can be used to select the appropriate adjuvant chemotherapy regimen for patients with D2-resected gastric cancer, particularly in stage III.
Ito T, Honma Y, Hirano H, et al.
S-1 Monotherapy After Failure of Platinum Plus 5-Fluorouracil Chemotherapy in Recurrent or Metastatic Esophageal Carcinoma.
Anticancer Res. 2019; 39(7):3931-3936 [PubMed] Related Publications
S-1 Monotherapy After Failure of Platinum Plus 5-Fluorouracil Chemotherapy in Recurrent or Metastatic Esophageal Carcinoma.
Anticancer Res. 2019; 39(7):3931-3936 [PubMed] Related Publications
BACKGROUND/AIM: Platinum plus 5-fluorouracil (FP) is a first-line regimen of palliative chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma (RM-ESCC). In this retrospective study, we evaluated the efficacy and safety of S-1 monotherapy as a salvage line treatment for RM-ESCC, focusing on the reasons for discontinuation of prior FP.
MATERIALS AND METHODS: The subjects of this study had RM-ESCC and received S-1 after failure of FP.
RESULTS: Eleven patients were enrolled. Nine patients were refractory and two were intolerant to prior FP. The median progression-free survival and overall survival time were 3.0 and 11.7 months, respectively. Overall response rate was 22.2% and disease control rate of the 11 patients was 36.4%. Median relative dose intensity of 5-FU was 100% (range=85-100%).
CONCLUSION: S-1 efficacy in RM-ESCC when given after FP was modest. Favorable OS may be attributed to good local control rather than to the efficacy of S-1 monotherapy.
MATERIALS AND METHODS: The subjects of this study had RM-ESCC and received S-1 after failure of FP.
RESULTS: Eleven patients were enrolled. Nine patients were refractory and two were intolerant to prior FP. The median progression-free survival and overall survival time were 3.0 and 11.7 months, respectively. Overall response rate was 22.2% and disease control rate of the 11 patients was 36.4%. Median relative dose intensity of 5-FU was 100% (range=85-100%).
CONCLUSION: S-1 efficacy in RM-ESCC when given after FP was modest. Favorable OS may be attributed to good local control rather than to the efficacy of S-1 monotherapy.
Sung PS, Yang K, Bae SH, et al.
Reduction of Intrahepatic Tumour by Hepatic Arterial Infusion Chemotherapy Prolongs Survival in Hepatocellular Carcinoma.
Anticancer Res. 2019; 39(7):3909-3916 [PubMed] Related Publications
Reduction of Intrahepatic Tumour by Hepatic Arterial Infusion Chemotherapy Prolongs Survival in Hepatocellular Carcinoma.
Anticancer Res. 2019; 39(7):3909-3916 [PubMed] Related Publications
BACKGROUND/AIM: This study aimed to identify the survival benefit of intrahepatic tumour control by hepatic arterial infusion chemotherapy (HAIC) in hepatocellular carcinoma (HCC) patients with portal vein tumour thrombus (PVTT) or extrahepatic metastasis.
PATIENTS AND METHODS: Between 2010 and 2017, a total of 187 consecutive patients with advanced HCC were treated with HAIC. The survival outcomes and response rates to HAIC were analysed.
RESULTS: The intrahepatic objective response (OR) rate of all enrolled patients was 18.7%. The survival outcome of patients with OR was significantly better from those without OR, irrespective of initial distant metastasis. Achievement of intrahepatic OR by HAIC and favourable liver function at the time of best response evaluation were two independent factors associated with better OS.
CONCLUSION: HAIC-induced intrahepatic tumour reduction significantly prolonged patient survival, irrespective of PVTT or initial distant metastasis.
PATIENTS AND METHODS: Between 2010 and 2017, a total of 187 consecutive patients with advanced HCC were treated with HAIC. The survival outcomes and response rates to HAIC were analysed.
RESULTS: The intrahepatic objective response (OR) rate of all enrolled patients was 18.7%. The survival outcome of patients with OR was significantly better from those without OR, irrespective of initial distant metastasis. Achievement of intrahepatic OR by HAIC and favourable liver function at the time of best response evaluation were two independent factors associated with better OS.
CONCLUSION: HAIC-induced intrahepatic tumour reduction significantly prolonged patient survival, irrespective of PVTT or initial distant metastasis.
Moon SU, Park Y, Park MG, et al.
Theragnosis by a miR-141-3p molecular beacon: simultaneous detection and sensitization of 5-fluorouracil resistant colorectal cancer cells through the activation of the TRIM13-associated apoptotic pathway.
Chem Commun (Camb). 2019; 55(52):7466-7469 [PubMed] Related Publications
Theragnosis by a miR-141-3p molecular beacon: simultaneous detection and sensitization of 5-fluorouracil resistant colorectal cancer cells through the activation of the TRIM13-associated apoptotic pathway.
Chem Commun (Camb). 2019; 55(52):7466-7469 [PubMed] Related Publications
We developed a molecular beacon targeting miR-141-3p, aberrantly increased in 5-fluorouracil-resistant colorectal cancer cells (R-CRCCs). It consists of a fluorophore-labeled oligonucleotide, antisense to miR-141-3p, and a quencher. It detected R-CRCCs and recovered the chemosensitivity of them to 5-fluorouracil by hybridization with miR-141-3p, which is applicable to cancer treatment.
Lin Z, Chen J, Guo Y
Efficacy of XELOX adjuvant chemotherapy for gastric mixed adenoneuroendocrine carcinoma: A case report.
Medicine (Baltimore). 2019; 98(23):e16000 [PubMed] Free Access to Full Article Related Publications
Efficacy of XELOX adjuvant chemotherapy for gastric mixed adenoneuroendocrine carcinoma: A case report.
Medicine (Baltimore). 2019; 98(23):e16000 [PubMed] Free Access to Full Article Related Publications
RATIONALE: Mixed adenoneuroendocrine carcinoma (MANEC) is a rare neoplasm, and consensus on the treatment is unavailable.
PATIENT CONCERN: A 60-year-old Chinese man presented with obstructive symptoms while eating and paroxysmal stomach pain for more than a month.
DIAGNOSIS: MANEC was diagnosed based on clinical manifestations, imaging findings, and pathological examinations.
INTERVENTIONS: The patient underwent radical gastrectomy and received XELOX adjuvant chemotherapy (oxaliplatin 200 mg day 1 + capecitabine 1.5 g twice a day) after surgery.
OUTCOMES: After 4 cycles of XELOX adjuvant chemotherapy were administered, abdominal computerized tomography and liver magnetic resonance showed liver metastasis.
LESSONS: The therapy of gastric MANEC is based on surgical operation, and adjuvant chemotherapy program has an important influence on its prognosis. Therefore, further studying the effectiveness of XELOX adjuvant chemotherapy for gastric MANEC is necessary.
PATIENT CONCERN: A 60-year-old Chinese man presented with obstructive symptoms while eating and paroxysmal stomach pain for more than a month.
DIAGNOSIS: MANEC was diagnosed based on clinical manifestations, imaging findings, and pathological examinations.
INTERVENTIONS: The patient underwent radical gastrectomy and received XELOX adjuvant chemotherapy (oxaliplatin 200 mg day 1 + capecitabine 1.5 g twice a day) after surgery.
OUTCOMES: After 4 cycles of XELOX adjuvant chemotherapy were administered, abdominal computerized tomography and liver magnetic resonance showed liver metastasis.
LESSONS: The therapy of gastric MANEC is based on surgical operation, and adjuvant chemotherapy program has an important influence on its prognosis. Therefore, further studying the effectiveness of XELOX adjuvant chemotherapy for gastric MANEC is necessary.
Chen J, Wang J
Efficacy and safety assessment of S-1-based regimens comparing to intravenous fluorouracil-based ones in Asian patients with metastatic colorectal carcinoma: A system review and meta-analysis.
Medicine (Baltimore). 2019; 98(23):e15999 [PubMed] Free Access to Full Article Related Publications
Efficacy and safety assessment of S-1-based regimens comparing to intravenous fluorouracil-based ones in Asian patients with metastatic colorectal carcinoma: A system review and meta-analysis.
Medicine (Baltimore). 2019; 98(23):e15999 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: We performed the present systematic review and meta-analysis to evaluate the efficacy and safety for S-1-based regimens comparing to intravenous fluorouracil-based ones in Asian patients with metastatic colorectal carcinoma (mCRC).
METHODS: Eligible prospective and controlled randomized clinical trials (RCT) were included, of which data were extracted by inclusion criteria and exclusion ones. Odds ratio (OR) and Hazard ratio (HR) of outcomes including objective response rate (ORR), disease control rate (DCR), progressive-free survival (PFS), overall survival (OS), and adverse events (AEs) were explored for the final analysis between the 2 groups.
RESULTS: A total of 23 eligible prospective, controlled RCTs including 2269 patients were enrolled for the pooled analysis. With the meta-analysis of available data, the results of the present research showed that there was no statistical difference on short-term efficacy including ORR (HR = 0.85, 95% CI: 0.71-1.01; P = .07) or DCR (HR = 0.88, 95% CI: 0.69-1.11; P = .27), as well as long-term efficacy including PFS (HR = 1.00, 95% CI: 0.90-1.11; P = .98) or OS (HR = 0.95, 95% CI: 0.82-1.10; P = .50). In addition, the incidences of AEs including leucopenia, neutropenia, and vomiting were statistically lower in S-1-based regimens comparing to intravenous fluorouracil-based ones, regardless of all grade or high grade (all P <.05). However, there were no significant differences detected among other AEs including anemia, thrombocytopenia, increased alanine aminotransferase concentration, stomatitis, anorexia, diarrhea, hand-foot syndrome (HFS), or sensory neuropathy among the 2 groups (all P >.05).
CONCLUSIONS: The present meta-analysis revealed that S-1-based regimens might be associated with comparable efficacy, as well as lower risk of leucopenia, neutropenia, and vomiting at all/high grade comparing to intravenous fluorouracil-based ones in Asian patients with mCRC.
METHODS: Eligible prospective and controlled randomized clinical trials (RCT) were included, of which data were extracted by inclusion criteria and exclusion ones. Odds ratio (OR) and Hazard ratio (HR) of outcomes including objective response rate (ORR), disease control rate (DCR), progressive-free survival (PFS), overall survival (OS), and adverse events (AEs) were explored for the final analysis between the 2 groups.
RESULTS: A total of 23 eligible prospective, controlled RCTs including 2269 patients were enrolled for the pooled analysis. With the meta-analysis of available data, the results of the present research showed that there was no statistical difference on short-term efficacy including ORR (HR = 0.85, 95% CI: 0.71-1.01; P = .07) or DCR (HR = 0.88, 95% CI: 0.69-1.11; P = .27), as well as long-term efficacy including PFS (HR = 1.00, 95% CI: 0.90-1.11; P = .98) or OS (HR = 0.95, 95% CI: 0.82-1.10; P = .50). In addition, the incidences of AEs including leucopenia, neutropenia, and vomiting were statistically lower in S-1-based regimens comparing to intravenous fluorouracil-based ones, regardless of all grade or high grade (all P <.05). However, there were no significant differences detected among other AEs including anemia, thrombocytopenia, increased alanine aminotransferase concentration, stomatitis, anorexia, diarrhea, hand-foot syndrome (HFS), or sensory neuropathy among the 2 groups (all P >.05).
CONCLUSIONS: The present meta-analysis revealed that S-1-based regimens might be associated with comparable efficacy, as well as lower risk of leucopenia, neutropenia, and vomiting at all/high grade comparing to intravenous fluorouracil-based ones in Asian patients with mCRC.
Nakagawa Y, Kuranaga Y, Tahara T, et al.
Induced miR-31 by 5-fluorouracil exposure contributes to the resistance in colorectal tumors.
Cancer Sci. 2019; 110(8):2540-2548 [PubMed] Free Access to Full Article Related Publications
Induced miR-31 by 5-fluorouracil exposure contributes to the resistance in colorectal tumors.
Cancer Sci. 2019; 110(8):2540-2548 [PubMed] Free Access to Full Article Related Publications
Drug resistance makes treatment difficult in cancers. The present study identifies and analyzes drug resistance-related miRNA in colorectal cancer. We established 4 types of 5-fluorouracil (5-FU)-resistant colon cancer cell lines in vitro and in vivo. We then analyzed the miRNA expression profile by miRNA array in these 4 cell lines, and identified the drug resistance-related miRNAs. We examined the expression levels of the identified miRNA in 112 colorectal tumor samples from the patients. We identified 12 possible miRNAs involved in 5-FU resistance by miRNA arrays. We then examined the relationship between miR-31, which was the most promising among them, and drug resistance. The ectopic expression of mimic miR-31 showed significant 5-FU resistance in the parental DLD-1 cells, while anti-miR-31 caused significant growth inhibition in DLD/F cells; that is, 5-FU-resistant colon cancer cell line DLD-1 under exposure to 5-FU. When we exposed high doses of 5-FU to parent or 5-FU-resistant cells, the expression levels of miR-31 were raised higher than those of controls. Notably, the expression levels of miR-31 were positively correlated with the grade of clinical stages of colorectal tumors. The protein expression levels of factors inhibiting hypoxia-inducible factor 1 were downregulated by transfection of mimic miR-31 into DLD-1 cells. This study provides evidence supporting the association of miR-31 with 5-FU drug resistance and clinical stages of colorectal tumors.
Verhave B, Goldberg M, Hashim P, Levitt J
Treatment of Arsenic-Induced Bowen’s Disease With Topical 5-Fluorouracil
J Drugs Dermatol. 2019; 18(5):477-479 [PubMed] Related Publications
Treatment of Arsenic-Induced Bowen’s Disease With Topical 5-Fluorouracil
J Drugs Dermatol. 2019; 18(5):477-479 [PubMed] Related Publications
Here, we present a case of arsenic-induced Bowen’s disease treated with a regimen consisting of topical 5-fluouracil and oral nicotinamide. The use of this therapy modality resulted in near complete resolution of all of the patient’s lesions except for those on her palms, soles, and scalp. Excellent wound care and treatment adherence were major factors contributing to the success of this treatment option. Our results ultimately provide an alternative approach to treating multiple arsenical keratoses in patients who are limited to a drug plan involving 5-FU and oral nicotinamide and who are able to be rigorously compliant with application of medication and wound care.
J Drugs Dermatol. 2019;18(5):477-479.
De Falco V, Natalicchio MI, Napolitano S, et al.
A case report of a severe fluoropyrimidine-related toxicity due to an uncommon DPYD variant.
Medicine (Baltimore). 2019; 98(21):e15759 [PubMed] Free Access to Full Article Related Publications
A case report of a severe fluoropyrimidine-related toxicity due to an uncommon DPYD variant.
Medicine (Baltimore). 2019; 98(21):e15759 [PubMed] Free Access to Full Article Related Publications
INTRODUCTION: Fluoropyrimidines such as 5-fluorouracil (5-FU) and its orally active prodrug, capecitabine, are widely used in the treatment of gastrointestinal cancer, including colorectal cancer. Dihydropyrimidine dehydrogenase (DPD) plays an important role in the 5-FU metabolism. Dihydropyrimidine dehydrogenase gene (DPYD) is a highly polymorphic gene with several hundreds of reported genetic variants and DPD activity levels vary considerably among individuals, with different 5-FU-related efficacy and toxicity. About 5% of the population is deficient in DPD enzyme activity. The most well studied DPYD variant is the IVS14+1G>A, also known as DPYD *2A. In this report, we present a case of a patient with a double heterozygote DPYD variant (DPYD activity score: 0,5 according to Clinical Pharmacogenetics Implementation Consortium) who experienced a severe fluoropyrimidine-related toxicity resolved without any consequence.
PATIENT CONCERNS: A 46-years-old Caucasian man with diagnosis of left colon adenocarcinoma underwent left hemicolectomy on July 2017: pT3 G3 N1c M0. According to the disease stage, he started an adjuvant therapy with XELOX using capecitabine at 50% of total dose, because of his DPYD IVS14+1G>A variant, detected before the treatment.
DIAGNOSIS: After few days, despite of this dose reduction, he experienced life-threatening adverse events such as mucositis G3, diarrhea G3, neutropenia G4, thrombocytopenia G4, and hyperbilirubinemia G3 according to Common Terminology Criteria for Adverse Events v 5.0.
INTERVENTIONS: As first, we set up an intensive rehydration therapy, antibiotic and antifungal prophylaxis, Granulocyte-Colony Stimulating Factors, and supportive blood transfusions. Additional genetic tests revealed a double heterozygote variant of DPYD gene (DPYD IVS14+1G>A and 2846A>T) which is a very rare situation and only 3 cases are described in literature, all of them concluded with patient's death.
OUTCOMES: After 3 weeks of intensive therapy, the patient was fully recovered. Furthermore, all the whole-body CT scans performed since discharge from the hospital until now, have confirmed no evidence of disease.
CONCLUSIONS: Recent studies demonstrated that screening strategy for the most common DPYD variants allowed for avoiding toxicities and saving money. This report underlines the importance of genotyping DPYD before treatment and emphasizes the role of genotype-guided dose individualization.
PATIENT CONCERNS: A 46-years-old Caucasian man with diagnosis of left colon adenocarcinoma underwent left hemicolectomy on July 2017: pT3 G3 N1c M0. According to the disease stage, he started an adjuvant therapy with XELOX using capecitabine at 50% of total dose, because of his DPYD IVS14+1G>A variant, detected before the treatment.
DIAGNOSIS: After few days, despite of this dose reduction, he experienced life-threatening adverse events such as mucositis G3, diarrhea G3, neutropenia G4, thrombocytopenia G4, and hyperbilirubinemia G3 according to Common Terminology Criteria for Adverse Events v 5.0.
INTERVENTIONS: As first, we set up an intensive rehydration therapy, antibiotic and antifungal prophylaxis, Granulocyte-Colony Stimulating Factors, and supportive blood transfusions. Additional genetic tests revealed a double heterozygote variant of DPYD gene (DPYD IVS14+1G>A and 2846A>T) which is a very rare situation and only 3 cases are described in literature, all of them concluded with patient's death.
OUTCOMES: After 3 weeks of intensive therapy, the patient was fully recovered. Furthermore, all the whole-body CT scans performed since discharge from the hospital until now, have confirmed no evidence of disease.
CONCLUSIONS: Recent studies demonstrated that screening strategy for the most common DPYD variants allowed for avoiding toxicities and saving money. This report underlines the importance of genotyping DPYD before treatment and emphasizes the role of genotype-guided dose individualization.
Xiao C, Qian J, Zheng Y, et al.
A phase II study of biweekly oxaliplatin plus S-1 combination chemotherapy as a first-line treatment for patients with metastatic or advanced gastric cancer in China.
Medicine (Baltimore). 2019; 98(20):e15696 [PubMed] Free Access to Full Article Related Publications
A phase II study of biweekly oxaliplatin plus S-1 combination chemotherapy as a first-line treatment for patients with metastatic or advanced gastric cancer in China.
Medicine (Baltimore). 2019; 98(20):e15696 [PubMed] Free Access to Full Article Related Publications
Oxaliplatin plus S-1 (SOX) was a first-line regimen for advanced gastric cancer. The continuous administration of S-1 for 3 weeks can result in unacceptable gastrointestinal and hematological toxicities. Therefore, an alternative regimen (administration of S-1 for 1-week followed by 1-week rest) is warrant for improved tolerability and noninferiority efficacy. We conducted a study to evaluate the efficacy and safety of biweekly SOX as the first-line chemotherapy in patients with metastatic or advanced gastric cancer in China.Patients with metastatic or previously untreated advanced gastric cancer were enrolled. Oxaliplatin was administered intravenously at a dose of 85 mg/m on day 1, while S-1 was administered orally in doses of 80, 100, or 120 mg/day depending on different body surface areas of <1.25 m, 1.25-1.5 m, or >1.5 m respectively; the total dose of S-1 was administered twice daily on days 1-7 followed by a 7-day rest. This schedule was repeated every 2 weeks until disease progressed or intolerable toxicity occurred.Forty-six patients (M/F = 33/13) received biweekly oxaliplatin and S-1 as first-line chemotherapy. A total of 257 treatment cycles were administered and the median number of cycles administered was 6. Thirty-six patients (78.3%) received second-line chemotherapy. The median progression free survival and median overall survival was 4.4 months (95% CI, 3.37-5.36 months) and 10.3 months (95% CI, 8.88-11.3 months), respectively. The 1-year and 2-year survival rate was 41% and 13%. The objective response rate was 30.43%, and the disease control rate was 76.08%. The observed adverse events of Grade 3/4 included were leukocytopenia (13.04%); anemia (13.04%); neutropenia (15.22%); neurological toxicity (2.17%); diarrhea (2.17%).The biweekly SOX regimen as first-line treatment was active and well tolerated in Chinese patients with metastatic or advanced gastric cancer.
Kaira K, Imai H, Souma R, et al.
An Exploratory Randomized Phase II Trial Comparing CDDP Plus S-1 With Bevacizumab and CDDP Plus Pemetrexed With Bevacizumab Against Patients With Advanced Non-squamous Non-small Cell Lung Cancer.
Anticancer Res. 2019; 39(5):2483-2491 [PubMed] Related Publications
An Exploratory Randomized Phase II Trial Comparing CDDP Plus S-1 With Bevacizumab and CDDP Plus Pemetrexed With Bevacizumab Against Patients With Advanced Non-squamous Non-small Cell Lung Cancer.
Anticancer Res. 2019; 39(5):2483-2491 [PubMed] Related Publications
BACKGROUND/AIM: It remains unclear which chemotherapeutic regimens are better for the addition of bevacizumab. We conducted an exploratory randomized phase II trial comparing first-line S-1 plus cisplatin with bevacizumab and pemetrexed plus cisplatin with bevacizumab in patients with advanced non-squamous non-small cell lung cancer (NSCLC).
PATIENTS AND METHODS: Chemotherapy-naïve patients received S-1 (80 mg/m
RESULTS: Forty-eight patients were enrolled in this study, and eligible patients were randomly assigned at 1:1 ratio to receive SCB (n=24) or PCB (n=24). The median number of chemotherapy and maintenance therapy for SCB and PCB was 4 (range, 1-6 cycles) and 4 (range, 2-6 cycles), and 5 (range, 0-39 cycles) and 5 (range, 0-28 cycles), respectively. The overall response rate (ORR) for PCB and SCB were 54.2% and 83.3%, respectively (p=0.06). The median progression-free survival (PFS) and overall survival (OS) for PCB and SCB were 406 and 351 days, (p=0.96), and 678 and 1190 days, respectively (p=0.23). The mild adverse events were observed in both regimens. TS expression was more predictive of the chemotherapeutic response in SCB compared to PCB, but not for PFS.
CONCLUSION: The combination regimen of SCB was identified as having a similar activity and tolerability to that of PCB in patients with advanced non-squamous NSCLC.
PATIENTS AND METHODS: Chemotherapy-naïve patients received S-1 (80 mg/m
RESULTS: Forty-eight patients were enrolled in this study, and eligible patients were randomly assigned at 1:1 ratio to receive SCB (n=24) or PCB (n=24). The median number of chemotherapy and maintenance therapy for SCB and PCB was 4 (range, 1-6 cycles) and 4 (range, 2-6 cycles), and 5 (range, 0-39 cycles) and 5 (range, 0-28 cycles), respectively. The overall response rate (ORR) for PCB and SCB were 54.2% and 83.3%, respectively (p=0.06). The median progression-free survival (PFS) and overall survival (OS) for PCB and SCB were 406 and 351 days, (p=0.96), and 678 and 1190 days, respectively (p=0.23). The mild adverse events were observed in both regimens. TS expression was more predictive of the chemotherapeutic response in SCB compared to PCB, but not for PFS.
CONCLUSION: The combination regimen of SCB was identified as having a similar activity and tolerability to that of PCB in patients with advanced non-squamous NSCLC.
Morita Y, Sakaguchi T, Kitajima R, et al.
Body weight loss after surgery affects the continuity of adjuvant chemotherapy for pancreatic cancer.
BMC Cancer. 2019; 19(1):416 [PubMed] Free Access to Full Article Related Publications
Body weight loss after surgery affects the continuity of adjuvant chemotherapy for pancreatic cancer.
BMC Cancer. 2019; 19(1):416 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Postoperative chemotherapy is beneficial for many pancreatic cancer patients. However, some patients require dose reduction or the discontinuation of adjuvant chemotherapy because of adverse treatment-related effects. In this study, we aimed to evaluate two main outcomes. First, we evaluated the clinicopathological factors affecting patient disease-free survival (DFS) and overall survival (OS) following upfront surgery. Second, we evaluated the factors that influence the continuity of adjuvant chemotherapy.
METHODS: Fifty-four patients with resected pancreatic cancer were enrolled. First, we evaluated the clinicopathological factors affecting postoperative survival using the Kaplan-Meier method and Cox regression method. Next, factors affecting the continuity of adjuvant chemotherapy were analyzed using multiple logistic regression analysis.
RESULTS: Univariate and multivariate analyses revealed that positive LN metastasis (HR (95% CI) 6.329 (2.381-16.95); p < 0.001) and relative dose intensity (RDI) < 80% for adjuvant chemotherapy (HR (95% CI) 5.154 (1.761-15.15); p = 0.003) were independent predictive factors for DFS. Regarding OS, extended dissection of the nerve plexus around the superior mesenteric artery (SMA) (HR (95% CI) 4.504 (1.721-11.76); p = 0.002), positive microscopic surgical margin (HR (95% CI) 5.565 (1.724-17.96); p = 0.004), and adjuvant chemotherapy of RDI < 80% (HR (95% CI) 3.534 (1.135-2.667); p = 0.029) were also independent predictive factors. Moreover, the level of RDI significantly correlated with DFS and OS. Multiple logistic regression analysis revealed that low RDI was significantly associated with postoperative body weight loss (BWL) ≥ 10%.
CONCLUSIONS: The following factors were significantly associated with poor survival: extended dissection of the nerve plexus around the SMA, lymph node metastasis, residual tumor, and RDI of the adjuvant chemotherapy. Patient's prognosis with adjuvant chemotherapy of RDI < 80% was worse. BWL ≥10% was the most important factor affecting the continuity of adjuvant chemotherapy. Perioperative nutritional intervention is necessary for patients who receive adjuvant chemotherapy for advanced pancreatic cancer.
METHODS: Fifty-four patients with resected pancreatic cancer were enrolled. First, we evaluated the clinicopathological factors affecting postoperative survival using the Kaplan-Meier method and Cox regression method. Next, factors affecting the continuity of adjuvant chemotherapy were analyzed using multiple logistic regression analysis.
RESULTS: Univariate and multivariate analyses revealed that positive LN metastasis (HR (95% CI) 6.329 (2.381-16.95); p < 0.001) and relative dose intensity (RDI) < 80% for adjuvant chemotherapy (HR (95% CI) 5.154 (1.761-15.15); p = 0.003) were independent predictive factors for DFS. Regarding OS, extended dissection of the nerve plexus around the superior mesenteric artery (SMA) (HR (95% CI) 4.504 (1.721-11.76); p = 0.002), positive microscopic surgical margin (HR (95% CI) 5.565 (1.724-17.96); p = 0.004), and adjuvant chemotherapy of RDI < 80% (HR (95% CI) 3.534 (1.135-2.667); p = 0.029) were also independent predictive factors. Moreover, the level of RDI significantly correlated with DFS and OS. Multiple logistic regression analysis revealed that low RDI was significantly associated with postoperative body weight loss (BWL) ≥ 10%.
CONCLUSIONS: The following factors were significantly associated with poor survival: extended dissection of the nerve plexus around the SMA, lymph node metastasis, residual tumor, and RDI of the adjuvant chemotherapy. Patient's prognosis with adjuvant chemotherapy of RDI < 80% was worse. BWL ≥10% was the most important factor affecting the continuity of adjuvant chemotherapy. Perioperative nutritional intervention is necessary for patients who receive adjuvant chemotherapy for advanced pancreatic cancer.
Zhu J, Zeng W, Ge L, et al.
Capecitabine versus 5-fluorouracil in neoadjuvant chemoradiotherapy of locally advanced rectal cancer: A meta-analysis.
Medicine (Baltimore). 2019; 98(17):e15241 [PubMed] Related Publications
Capecitabine versus 5-fluorouracil in neoadjuvant chemoradiotherapy of locally advanced rectal cancer: A meta-analysis.
Medicine (Baltimore). 2019; 98(17):e15241 [PubMed] Related Publications
BACKGROUND: The differences in efficacy between capecitabine and 5-fuorouracil (5-FU) in neoadjuvant chemoradiotherapy (CRT) of locally advanced rectal cancer (LARC) are not well recognized. We performed this meta-analysis to analyze the effect of capecitabine and 5-FU on neoadjuvant CRT to more accurately understand the differences between the 2 drugs.
METHODS: MEDLINE, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and Wanfang Database were performed to identify all published studies investigating the efficacy of capecitabine in neoadjuvant CRT of LARC versus 5-FU before August, 2017. Primary endpoint was the odds ratio (OR) for improving pathological complete response (pCR) rate of patients with LARC. Secondary endpoints were the ORs of efficiency for downstaging tumor and increasing R0 resection in patients with LARC. Safety analyses were also performed. The OR was the principal measurement of effect, which was calculated as capecitabine group versus 5-FU group, and was presented as a point estimate with 95% confidence intervals (CIs). All calculations and statistical tests were performed using RevMan 5.3 software.
RESULTS: In all, 2916 patients with LARC enrolled in the 10 studies were divided into capecitabine group (n = 1451) and 5-FU group (n = 1465). The meta-analysis showed that capecitabine improved pCR (OR 1.34, 95% CI 1.10-1.63), and R0 resection rate (OR 1.92, 95% CI 1.10-3.36). There were no statistically significant differences either in overall downstaging rate (OR 1.31, 95% CI 0.79-2.16) or in the tumor downstaging rate (OR 1.24, 95% CI 0.79-1.92), but there was a significant difference of the nodal downstaging rate between the 2 groups (OR 1.68, 95% CI 1.11-2.54). There was no statistically significant difference in sphincter preservation rate between the 2 groups (OR 1.36, 95% CI 0.96-1.92). No obvious safety concerns about mortality and complications were raised in these studies. There were no statistically significant differences in 3-year disease-free-survival (OR 1.29, 95% CI 0.75-2.20), and in grade 3 to 4 acute toxicity during CRT (OR 0.63, 95% CI 0.31-1.30).
CONCLUSIONS: Compared with 5-FU-based neoadjuvant CRT, capecitabine-based neoadjuvant CRT can safely improve pCR, nodal down-staging, ad R0 resection of patients with LARC.
METHODS: MEDLINE, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and Wanfang Database were performed to identify all published studies investigating the efficacy of capecitabine in neoadjuvant CRT of LARC versus 5-FU before August, 2017. Primary endpoint was the odds ratio (OR) for improving pathological complete response (pCR) rate of patients with LARC. Secondary endpoints were the ORs of efficiency for downstaging tumor and increasing R0 resection in patients with LARC. Safety analyses were also performed. The OR was the principal measurement of effect, which was calculated as capecitabine group versus 5-FU group, and was presented as a point estimate with 95% confidence intervals (CIs). All calculations and statistical tests were performed using RevMan 5.3 software.
RESULTS: In all, 2916 patients with LARC enrolled in the 10 studies were divided into capecitabine group (n = 1451) and 5-FU group (n = 1465). The meta-analysis showed that capecitabine improved pCR (OR 1.34, 95% CI 1.10-1.63), and R0 resection rate (OR 1.92, 95% CI 1.10-3.36). There were no statistically significant differences either in overall downstaging rate (OR 1.31, 95% CI 0.79-2.16) or in the tumor downstaging rate (OR 1.24, 95% CI 0.79-1.92), but there was a significant difference of the nodal downstaging rate between the 2 groups (OR 1.68, 95% CI 1.11-2.54). There was no statistically significant difference in sphincter preservation rate between the 2 groups (OR 1.36, 95% CI 0.96-1.92). No obvious safety concerns about mortality and complications were raised in these studies. There were no statistically significant differences in 3-year disease-free-survival (OR 1.29, 95% CI 0.75-2.20), and in grade 3 to 4 acute toxicity during CRT (OR 0.63, 95% CI 0.31-1.30).
CONCLUSIONS: Compared with 5-FU-based neoadjuvant CRT, capecitabine-based neoadjuvant CRT can safely improve pCR, nodal down-staging, ad R0 resection of patients with LARC.
Francipane MG, Bulanin D, Lagasse E
Establishment and Characterization of 5-Fluorouracil-Resistant Human Colorectal Cancer Stem-Like Cells: Tumor Dynamics under Selection Pressure.
Int J Mol Sci. 2019; 20(8) [PubMed] Free Access to Full Article Related Publications
Establishment and Characterization of 5-Fluorouracil-Resistant Human Colorectal Cancer Stem-Like Cells: Tumor Dynamics under Selection Pressure.
Int J Mol Sci. 2019; 20(8) [PubMed] Free Access to Full Article Related Publications
5-Fluorouracil (5-FU) remains the gold standard of first-line treatment for colorectal cancer (CRC). Although it may initially debulk the tumor mass, relapses frequently occur, indicating the existence of cancer cells that are therapy-resistant and are capable of refueling tumor growth. To identify mechanisms of drug resistance, CRC stem-like cells were subjected to long-term 5-FU selection using either intermittent treatment regimen with the IC50 drug dose or continuous treatment regimen with escalating drug doses. Parental cancer cells were cultivated in parallel. Real-time PCR arrays and bioinformatic tools were used to investigate gene expression changes. We found the first method selected for cancer cells with more aggressive features. We therefore transplanted these cancer cells or parental cells in mice, and again, found that not only did the 5-FU-selected cancer cells generate more aggressive tumors with respect to their parental counterpart, but they also showed a different gene expression pattern as compared to what we had observed in vitro, with
Yasui H, Kawakami T, Kashiwagi H, et al.
Pharmacokinetics of S-1 monotherapy in plasma and in tears for gastric cancer patients.
Int J Clin Oncol. 2019; 24(6):660-665 [PubMed] Free Access to Full Article Related Publications
Pharmacokinetics of S-1 monotherapy in plasma and in tears for gastric cancer patients.
Int J Clin Oncol. 2019; 24(6):660-665 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: S-1 is an oral anticancer drug composed of tegafur (FT), which is a prodrug of 5-FU, 5-chloro-2,4-dihydroxypyridine (CDHP), and potassium oxonate. Recently, some studies have been reported on watering eyes caused by S-1. However, the mechanism of watering eyes caused by S-1 is still unclear. The aim of this study was to investigate the correlation between tears and plasma concentrations of FT, 5-FU, and CDHP, which are components and active modulator of S-1.
METHODS: We prospectively investigated the pharmacokinetics (PK) of FT, 5-FU, and CDHP in plasma and in tears of gastric cancer patients who were treated with S-1 monotherapy at the dose of 80 mg/m
RESULTS: Total of eight patients were enrolled. All the FT, 5-FU and CDHP were detected both in plasma and in tears, and their PK parameters were measured. There was a positive correlation between the concentrations of FT, 5-FU and CDHP in the plasma and those in the tears on day 1 and day 14 (correlation coefficients r, right eye/left eye: r = 0.882/0.878, 0.877/0.890, and 0.885/0.878, respectively).
CONCLUSION: There was a positive correlation between the concentrations of FT, 5-FU and CDHP in the plasma and those in the tears. The result is expected to facilitate the further investigation into the causes of watering eyes and the establishment of the effective methods for the prevention and the treatment.
METHODS: We prospectively investigated the pharmacokinetics (PK) of FT, 5-FU, and CDHP in plasma and in tears of gastric cancer patients who were treated with S-1 monotherapy at the dose of 80 mg/m
RESULTS: Total of eight patients were enrolled. All the FT, 5-FU and CDHP were detected both in plasma and in tears, and their PK parameters were measured. There was a positive correlation between the concentrations of FT, 5-FU and CDHP in the plasma and those in the tears on day 1 and day 14 (correlation coefficients r, right eye/left eye: r = 0.882/0.878, 0.877/0.890, and 0.885/0.878, respectively).
CONCLUSION: There was a positive correlation between the concentrations of FT, 5-FU and CDHP in the plasma and those in the tears. The result is expected to facilitate the further investigation into the causes of watering eyes and the establishment of the effective methods for the prevention and the treatment.
Ulusakarya A, Teyar N, Karaboué A, et al.
Patient-tailored FOLFIRINOX as first line treatment of patients with advanced pancreatic adenocarcinoma.
Medicine (Baltimore). 2019; 98(16):e15341 [PubMed] Free Access to Full Article Related Publications
Patient-tailored FOLFIRINOX as first line treatment of patients with advanced pancreatic adenocarcinoma.
Medicine (Baltimore). 2019; 98(16):e15341 [PubMed] Free Access to Full Article Related Publications
FOLFIRINOX is one of the most effective reference regimens in the 1st line treatment of locally advanced (LA) and metastatic pancreatic cancer (mPC), despite its high toxicity. We evaluated our real-life experience with "patient-tailored intent to treat FOLFIRINOX" in patients with LA or mPC compared to other reports along with the pivotal phase III trial.We analyzed data from all consecutive patients with pancreatic ductal adenocarcinoma treated with dose-modified FOLFIRINOX in 2016 at Paul Brousse University Hospital. Irinotecan was administered whenever initial serum bilirubin was <1.5 × upper limit of normal. Oxaliplatin was stopped for severe sensory neuropathy. Initial dose reductions were made according to patient profile (eg, age, comorbidities) and later due to toxicity. The treatment was continued until surgery or disease progression. Endpoints were time to progression (TTP), overall survival (OS), objective response rate (ORR), and secondary complete resection (R0R1).Thirty-seven patients with unresectable LA or mPC received patient-tailored FOLFIRINOX as 1st line chemotherapy. There were 22 male (59%) and 15 female patients (41%) aged 44 to 81 years with LA (18 patients, 49%) and mPC (19 patients, 51%). They had World Health Organization-performance status of 0 (59%) or 1 (41%). A total of 384 cycles were administered. Median dose intensities (mg/m/w) were 28.9 for oxaliplatin, 56.8 for irinotecan, and 886.2 for 5-fluorouracil. Thirty-four patients were assessed for response; ORR and disease control rates were 47% and 85%, respectively. R0R1 rate was 30%. Median TTP and OS were 9.6 and 14.6 months. LA disease was associated with significantly longer TTP and OS (P < .001).FOLFIRINOX with patient-tailored dose adaptations seems to offer better results in patients with advanced PC. This approach in the neoadjuvant setting results in a macroscopic R0R1 in 61% of patients with initially unresectable disease. It deserves prospective evaluation to further improve outcomes in the management of advanced PC.
Ghosh S, Pal A, Ray M
Methylglyoxal in combination with 5-Fluorouracil elicits improved chemosensitivity in breast cancer through apoptosis and cell cycle inhibition.
Biomed Pharmacother. 2019; 114:108855 [PubMed] Related Publications
Methylglyoxal in combination with 5-Fluorouracil elicits improved chemosensitivity in breast cancer through apoptosis and cell cycle inhibition.
Biomed Pharmacother. 2019; 114:108855 [PubMed] Related Publications
The anti-carcinogenic effect of Methylglyoxal (MG) is well established. It generally targets malignant cells by affecting glycolysis and mitochondrial respiration with minimum or no toxicity to normal cells. In an initial study we have reported that MG can synergistically act with 5-Fluorouracil (5-FU) to decreases the number of MCF-7 breast cancer cells (Ghosh S, Pal A, Ray M, 2017). This finding prompted us to study the combination effect of MG and 5-FU extensively in both in vitro and in vivo. Induction of cell apoptosis and cell cycle arrest was systematically studied to reveal the mechanisms of synergy between 5-FU and MG. Our present study revealed that MG can synergistically act with 5-FU and can cause cell death via apoptosis and generated reactive oxygen species (ROS) in MCF-7 cells. Combination of 5-FU and MG resulted in more potent apoptosis induction as revealed by fluorescence microscopy using Hoechst 33342. In comparison to single drug treatment, the co-treatment also increased the number of cells in G0/G1 phase by downregulating the expression of CDK4 and CDK6 as compared to single drug treatment. Levels of Caspase 9 and poly (ADP-ribose) polymerase (PARP) were higher in combination treatment as compared to single drug treatment. These results clearly showed that 5-FU is more effective at lower doses in presence of MG in MCF-7 cells. In case of in vivo studies treatment of EAC (Ehrlich Ascites Carcinoma) bearing mice with MG in combination with 5-FU at various doses, demonstrated the same synergistic effect of MG with 5-FU. The combination study also exhibited tumor regression in BALB/c mouse 4T1 breast tumor model as well. We also clearly demonstrated that MG can decrease the cytotoxic side effects of 5-FU as indicated with acute and chronic toxicity studies and other biochemical analyses of blood and histological studies. Taken together, our results revealed that MG could be a potential candidate for combination therapy to reduce the toxicity burden of 5-FU without any toxic impact on host cells.
Larsen FO, Jensen BV, Nørgaard HH, et al.
Intrahepatic Oxaliplatin and Systemic 5-FU +/- Cetuximab in Chemo-Naïve Patients with Liver Metastases from Colorectal Cancer.
Oncology. 2019; 96(6):299-308 [PubMed] Related Publications
Intrahepatic Oxaliplatin and Systemic 5-FU +/- Cetuximab in Chemo-Naïve Patients with Liver Metastases from Colorectal Cancer.
Oncology. 2019; 96(6):299-308 [PubMed] Related Publications
BACKGROUND: In case of response to chemotherapy, unresectable liver metastases from colorectal cancer can be converted to resectable and thereby obtain a chance of cure. The primary aim of this trial was to evaluate the response rate with intrahepatic oxaliplatin in combination with systemic 5-FU +/- cetuximab. Secondary aims were to evaluate the conversion rate from unresectable to resectable liver metastases, median progression-free survival, median overall survival, and toxicity.
METHODS: Forty-five chemo-naïve patients with liver metastases from colorectal cancer were treated in a prospective phase II trial. Calcium folinate and 5-FU were delivered systemically while oxaliplatin was delivered alternating between systemic and intrahepatic administration. When oxaliplatin was delivered intrahepatic-ally, infusion time was reduced to 10 min followed by embolic material. In patients with KRAS wild-type tumors, cetuximab was added.
RESULTS: The treatment was well tolerated and only pain in the liver and a mild increase in liver enzymes were observed after intrahepatic oxaliplatin. The patients obtained a response rate of 82%. Further, 58% converted from having unresectable to resectable liver metastases. The median overall survival and progression-free survival were 38.7 months (95% confidence interval [CI] 33.0-44.3) and 12.9 months (95% CI 10.2-15.6), respectively.
CONCLUSIONS: Intrahepatic infusion of oxaliplatin in 10 min with systemic 5-FU to patients with chemo-naïve colorectal cancer is feasible and with low toxicity. A high response rate and long median overall survival were obtained.
METHODS: Forty-five chemo-naïve patients with liver metastases from colorectal cancer were treated in a prospective phase II trial. Calcium folinate and 5-FU were delivered systemically while oxaliplatin was delivered alternating between systemic and intrahepatic administration. When oxaliplatin was delivered intrahepatic-ally, infusion time was reduced to 10 min followed by embolic material. In patients with KRAS wild-type tumors, cetuximab was added.
RESULTS: The treatment was well tolerated and only pain in the liver and a mild increase in liver enzymes were observed after intrahepatic oxaliplatin. The patients obtained a response rate of 82%. Further, 58% converted from having unresectable to resectable liver metastases. The median overall survival and progression-free survival were 38.7 months (95% confidence interval [CI] 33.0-44.3) and 12.9 months (95% CI 10.2-15.6), respectively.
CONCLUSIONS: Intrahepatic infusion of oxaliplatin in 10 min with systemic 5-FU to patients with chemo-naïve colorectal cancer is feasible and with low toxicity. A high response rate and long median overall survival were obtained.
Yoshida Y, Beppu T, Kinoshita K, et al.
Five-year Recurrence-free Survival After Surgery Followed by Oral Chemotherapy for Gastric Cancer With Portal Vein Tumor Thrombosis.
Anticancer Res. 2019; 39(4):2233-2238 [PubMed] Related Publications
Five-year Recurrence-free Survival After Surgery Followed by Oral Chemotherapy for Gastric Cancer With Portal Vein Tumor Thrombosis.
Anticancer Res. 2019; 39(4):2233-2238 [PubMed] Related Publications
Gastric cancer with portal vein tumor thrombosis (GC-PVTT) is a rare condition with a very poor prognosis. A 64-year-old man with GC-PVTT was admitted to our hospital. His carcinoembryonic antigen level was slightly elevated (17.4 ng/ml). Upper gastrointestinal endoscopy showed a type-2 gastric lesion (45 mm × 40 mm) in the gastric antrum. The PVTT originated from the main gastric tumor and continued to the superior mesenteric vein. Fluorodeoxyglucose-positron emission tomography showed high uptake both by the main tumor and PVTT. A distal gastrectomy with D2 lymphadenectomy was performed with simultaneous removal of the PVTT. Pathological examination showed a poorly differentiated adenocarcinoma with neuroendocrine differentiation. Adjuvant chemotherapy with S-1 was administered for 1 year. The patient survived for >5 years with no recurrence. Surgical gastrectomy and complete removal of the PVTT followed by S-1 chemotherapy could be a treatment option that offers improved long-term survival for patients with GC-PVTT.
Liu JB, Jian T, Yue C, et al.
Chemo-resistant Gastric Cancer Associated Gene Expression Signature: Bioinformatics Analysis Based on Gene Expression Omnibus.
Anticancer Res. 2019; 39(4):1689-1698 [PubMed] Related Publications
Chemo-resistant Gastric Cancer Associated Gene Expression Signature: Bioinformatics Analysis Based on Gene Expression Omnibus.
Anticancer Res. 2019; 39(4):1689-1698 [PubMed] Related Publications
BACKGROUND/AIM: This study aimed to identify biomarkers for predicting the prognosis of advanced gastric cancer patients who received docetaxel, cisplatin, and S-1 (DCS).
MATERIALS AND METHODS: Gene expression profiles were obtained from the Gene Expression Omnibus database (GSE31811). Gene-Ontology-enrichment and KEGG-pathway analysis were used for evaluating the biological functions of differentially-expressed genes. Protein-protein interaction (PPI) network and Kaplan-Meier survival analyses were employed to assess the prognostic values of hub genes.
RESULTS: A total of 1,486 differentially expressed genes (DEGs) were identified, including 13 up-regulated and 1,473 down-regulated genes. KEGG pathways such as metabolic pathways, cell adhesion molecules (CAMs), PI3K-Akt signaling pathway and pathways in cancer were significantly represented. In the PPI network, the top ten hub genes ranked by degree were GNG7, PLCB1, CALML5, FGFR4, GRB2, JAK3, ADCY7, ADCY9, GNAS and KDR. Five DEGs, including ANTXR1, EFNA5, GAMT, E2F2 and NRCAM, were associated with relapse-free survival and overall survival.
CONCLUSION: ANTXR1, EFNA5, GAMT, E2F2 and NRCAM are potential biomarkers and therapeutic targets for DCS treatment in GC.
MATERIALS AND METHODS: Gene expression profiles were obtained from the Gene Expression Omnibus database (GSE31811). Gene-Ontology-enrichment and KEGG-pathway analysis were used for evaluating the biological functions of differentially-expressed genes. Protein-protein interaction (PPI) network and Kaplan-Meier survival analyses were employed to assess the prognostic values of hub genes.
RESULTS: A total of 1,486 differentially expressed genes (DEGs) were identified, including 13 up-regulated and 1,473 down-regulated genes. KEGG pathways such as metabolic pathways, cell adhesion molecules (CAMs), PI3K-Akt signaling pathway and pathways in cancer were significantly represented. In the PPI network, the top ten hub genes ranked by degree were GNG7, PLCB1, CALML5, FGFR4, GRB2, JAK3, ADCY7, ADCY9, GNAS and KDR. Five DEGs, including ANTXR1, EFNA5, GAMT, E2F2 and NRCAM, were associated with relapse-free survival and overall survival.
CONCLUSION: ANTXR1, EFNA5, GAMT, E2F2 and NRCAM are potential biomarkers and therapeutic targets for DCS treatment in GC.
Han L, Wei ZX, Lv YF, Jiang AY
Efficacy of carboplatin plus S-1 for the treatment of non-small cell lung cancer: A protocol for a systematic review of randomized controlled trial.
Medicine (Baltimore). 2019; 98(14):e15099 [PubMed] Free Access to Full Article Related Publications
Efficacy of carboplatin plus S-1 for the treatment of non-small cell lung cancer: A protocol for a systematic review of randomized controlled trial.
Medicine (Baltimore). 2019; 98(14):e15099 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Non-small cell lung cancer (NSCLC) is the most common lung cancer. Numerous clinical studies have reported that the combination of carboplatin and S-1 (CS) can be used to treat NSCLC effectively. However, no systematic review has been conducted to assess its efficacy and safety for NSCLC. This systematic review aims to evaluate the efficacy and safety of CS for treatment of patients with NSCLC.
METHODS: This study will retrieve the following electronic databases from inception to the February 1, 2019: Cochrane Library, EMBASE, MEDILINE, CINAHL, AMED, and 4 Chinese databases without any language limitations. This systematic review will include randomized controlled trials (RCTs) and case-control studies for assessing the efficacy and safety of CS for the treatment of NSCLC. Cochrane risk of bias will be used as methodological quality assessment for each qualified study. The RevMan V.5.3 software will be utilized to synthesize the data and conduct the meta-analysis if it is allowed. The data will be pooled by using the random-effects model or fixed-effects model.
RESULTS: The primary outcome is overall response rate. The secondary outcomes are overall survival, progression-free survival, the disease control rate, and any adverse events.
CONCLUSION: It will provide latest evidence to determine the efficacy and safety of CS for treatment of patients with NSCLC.
ETHICS AND DISSEMINATION: No research ethic approval is needed in this study because this study will not analyze individual patient data. The results are expected to disseminate through peer-reviewed journals.
SYSTEMATIC REVIEW REGISTRATION: PROSPERO CRD42019124860.
METHODS: This study will retrieve the following electronic databases from inception to the February 1, 2019: Cochrane Library, EMBASE, MEDILINE, CINAHL, AMED, and 4 Chinese databases without any language limitations. This systematic review will include randomized controlled trials (RCTs) and case-control studies for assessing the efficacy and safety of CS for the treatment of NSCLC. Cochrane risk of bias will be used as methodological quality assessment for each qualified study. The RevMan V.5.3 software will be utilized to synthesize the data and conduct the meta-analysis if it is allowed. The data will be pooled by using the random-effects model or fixed-effects model.
RESULTS: The primary outcome is overall response rate. The secondary outcomes are overall survival, progression-free survival, the disease control rate, and any adverse events.
CONCLUSION: It will provide latest evidence to determine the efficacy and safety of CS for treatment of patients with NSCLC.
ETHICS AND DISSEMINATION: No research ethic approval is needed in this study because this study will not analyze individual patient data. The results are expected to disseminate through peer-reviewed journals.
SYSTEMATIC REVIEW REGISTRATION: PROSPERO CRD42019124860.
Liang D, Yang B
Short-term efficacy of oral low-dose Tegafur chemotherapy after transarterial chemoembolization in primary hepatic carcinoma.
J BUON. 2019 Jan-Feb; 24(1):171-177 [PubMed] Related Publications
Short-term efficacy of oral low-dose Tegafur chemotherapy after transarterial chemoembolization in primary hepatic carcinoma.
J BUON. 2019 Jan-Feb; 24(1):171-177 [PubMed] Related Publications
PURPOSE: To observe the short-term efficacy of oral low-dose Tegafur chemotherapy after transarterial chemoembolization (TACE) in primary hepatic carcinoma (PHC).
METHODS: 120 PHC patients undergoing TACE treatment for the first were randomly divided into the Tegafur group and the TACE group. Patients in TACE group received TACE only, whereas those in the Tegafur group received TACE and postoperative oral low-dose Tegafur chemotherapy. All patients were followed up for 4 to 20 months. Clinical efficacy, liver function changes, progression-free survival (PFS), and adverse reactions were compared between the two groups.
RESULTS: The disease control rate (DCR) and clinical benefit rate (CBR) of the Tegafur group were significantly higher than those of TACE group (p<0.05). Moreover, higher PFS was found in the Tegafur group than that of the TACE group after 18 months of follow-up (p<0.05). Before treatment, serum levels of ALT, AST, TBIL and DBIL in the two groups were not statistically significant (p>0.05). After treatment, the above-mentioned indicators were remarkably increased in both groups. In particular, the indicators were lower in the Tegafur group than those of the TACE group (p<0.05).
CONCLUSIONS: TACE combined with low-dose Tegafur for treating PHC can slow down the tumor progression and prolong the PFS. This approach is safe and effective.
METHODS: 120 PHC patients undergoing TACE treatment for the first were randomly divided into the Tegafur group and the TACE group. Patients in TACE group received TACE only, whereas those in the Tegafur group received TACE and postoperative oral low-dose Tegafur chemotherapy. All patients were followed up for 4 to 20 months. Clinical efficacy, liver function changes, progression-free survival (PFS), and adverse reactions were compared between the two groups.
RESULTS: The disease control rate (DCR) and clinical benefit rate (CBR) of the Tegafur group were significantly higher than those of TACE group (p<0.05). Moreover, higher PFS was found in the Tegafur group than that of the TACE group after 18 months of follow-up (p<0.05). Before treatment, serum levels of ALT, AST, TBIL and DBIL in the two groups were not statistically significant (p>0.05). After treatment, the above-mentioned indicators were remarkably increased in both groups. In particular, the indicators were lower in the Tegafur group than those of the TACE group (p<0.05).
CONCLUSIONS: TACE combined with low-dose Tegafur for treating PHC can slow down the tumor progression and prolong the PFS. This approach is safe and effective.
Huang J, Xu B, Liu Y, et al.
Irinotecan plus S-1 versus S-1 in patients with previously treated recurrent or metastatic esophageal cancer (ESWN 01): a prospective randomized, multicenter, open-labeled phase 3 trial.
Cancer Commun (Lond). 2019; 39(1):16 [PubMed] Free Access to Full Article Related Publications
Irinotecan plus S-1 versus S-1 in patients with previously treated recurrent or metastatic esophageal cancer (ESWN 01): a prospective randomized, multicenter, open-labeled phase 3 trial.
Cancer Commun (Lond). 2019; 39(1):16 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: The benefit of systemic treatments in esophageal squamous cell carcinoma (ESCC) which has progressed after chemotherapy is still uncertain and optimal regimens based on randomized trials have not yet been established. We aimed to compare the efficacy of irinotecan plus S-1 with S-1 monotherapy in recurrent or metastatic ESCC patients who had resistance to platinum- or taxane-based chemotherapy.
METHODS: We conducted a prospective randomized, multicenter, open-label, phase 3 trial in 15 centers across China. Eligible patients were adults with histologically confirmed recurrent or metastatic ESCC, and were randomly assigned (ratio, 1:1) to receive either irinotecan plus S-1 (intravenous infusion of irinotecan [160 mg/m
RESULTS: Between December 23, 2014 and July 25, 2016, we screened 148 patients and randomly assigned 123 patients to receive either irinotecan plus S-1 regimen (n = 61) or S-1 monotherapy (n = 62). After a median follow-up of 29.2 months (95% confidence interval [CI] 17.5-40.9 months), the median PFS was significantly longer in the irinotecan plus S-1 group than in the S-1 monotherapy group (3.8 months [95% CI 2.9-4.3 months] vs. 1.7 months [95% CI 1.4-2.7 months], hazard ratio = 0.58, 95% CI 0.38-0.86, P = 0.006). The objective response rates were 24.6% in the irinotecan plus S-1 group and 9.7% in the S-1 monotherapy group (P = 0.002). The patients in the irinotecan plus S-1 group presented with increased rates of grade 3-4 leukopenia (16.4% vs. 0%), neutropenia (14.8% vs. 1.6%), and nausea (4.9% vs. 0%). No significant difference in grade 3-4 diarrhea and no treatment-related deaths were observed in both groups.
CONCLUSIONS: The combination of irinotecan with S-1 was similarly tolerable but significantly prolonged PFS compared to S-1 monotherapy as a second- or third-line treatment in patients with recurrent or metastatic ESCC. Clinical Trial Registration NCT02319187. Registered on December 9, 2014.
METHODS: We conducted a prospective randomized, multicenter, open-label, phase 3 trial in 15 centers across China. Eligible patients were adults with histologically confirmed recurrent or metastatic ESCC, and were randomly assigned (ratio, 1:1) to receive either irinotecan plus S-1 (intravenous infusion of irinotecan [160 mg/m
RESULTS: Between December 23, 2014 and July 25, 2016, we screened 148 patients and randomly assigned 123 patients to receive either irinotecan plus S-1 regimen (n = 61) or S-1 monotherapy (n = 62). After a median follow-up of 29.2 months (95% confidence interval [CI] 17.5-40.9 months), the median PFS was significantly longer in the irinotecan plus S-1 group than in the S-1 monotherapy group (3.8 months [95% CI 2.9-4.3 months] vs. 1.7 months [95% CI 1.4-2.7 months], hazard ratio = 0.58, 95% CI 0.38-0.86, P = 0.006). The objective response rates were 24.6% in the irinotecan plus S-1 group and 9.7% in the S-1 monotherapy group (P = 0.002). The patients in the irinotecan plus S-1 group presented with increased rates of grade 3-4 leukopenia (16.4% vs. 0%), neutropenia (14.8% vs. 1.6%), and nausea (4.9% vs. 0%). No significant difference in grade 3-4 diarrhea and no treatment-related deaths were observed in both groups.
CONCLUSIONS: The combination of irinotecan with S-1 was similarly tolerable but significantly prolonged PFS compared to S-1 monotherapy as a second- or third-line treatment in patients with recurrent or metastatic ESCC. Clinical Trial Registration NCT02319187. Registered on December 9, 2014.
Amasya G, Aksu B, Badilli U, et al.
QbD guided early pharmaceutical development study: Production of lipid nanoparticles by high pressure homogenization for skin cancer treatment.
Int J Pharm. 2019; 563:110-121 [PubMed] Related Publications
QbD guided early pharmaceutical development study: Production of lipid nanoparticles by high pressure homogenization for skin cancer treatment.
Int J Pharm. 2019; 563:110-121 [PubMed] Related Publications
This research attempts to bring together the positive aspects of lipid nanoparticles and Quality by Design (QbD) approach for developing a novel drug delivery system for skin cancers and aktinic keratosis. Lipid nanoparticles which is one of the most efficacious options for topical treatment of skin diseases were prepared due to their ability to overcome the complex structure of skin barrier and to enhance the skin penetration. Since the formulation development contains complex variables of active ingredients, raw materials or production method; all the variables of the product should be elaborated. QbD approach which refers to design and develop formulations and manufacturing processes to maintain the prescribed product quality was also successfully adopted to achieve a time- and cost-saving process ensuring a high-quality product. 5-Fluorouracil (5-FU) loaded lipid nanoparticles, both solid lipid nanoparticles and nanostructured lipid carriers, were developed and characterized by following QbD steps. Optimal lipid nanoparticle formulation with guaranteed quality which was within the design space has been reached through the artificial neural networks. The optimal lipid nanoparticle formulation which is a NLC formulation with a mean particle size of 205,8 ± 9,34 nm, narrow size distribution (0.279 ± 0.01) and negative zeta potantial -30,20 ± 0,92 was produced by high pressure homogenization method. Cytotoxicity profiles of the optimal NLC was determined by cell culture studies on epidermoid carcinoma cells and human keratinocyte cells. Optimal NLC showed significantly higher anticancer effect on epidermoid carcinoma cells than free 5-FU and also less cytotoxicity towards human keratinocyte cells. Optimal NLC was formulated in hydrogel formulation for ease of application which has suitable occlusive and mechanical properties, viscocity and pH for patient complience. The cumulative amount of 5-FU in dermal tissues of rat skin was found 20.11 ± 2.14 μg/cm2 and 9.73 ± 0.87 μg/cm2 after application of NLC enriched hydrogel and 5-FU hydrogel respectively. In conclusion, this study showed that a time and cost saving process ensuring a high-quality product can be obtained by QbD guided formulation development study with the help of artificial neural networks. A novel semisolid dosage form enriched by NLC which is promising for topical treatment of skin cancers was developed.
Lim H, Kim SY, Lee E, et al.
Sex-Dependent Adverse Drug Reactions to 5-Fluorouracil in Colorectal Cancer.
Biol Pharm Bull. 2019; 42(4):594-600 [PubMed] Related Publications
Sex-Dependent Adverse Drug Reactions to 5-Fluorouracil in Colorectal Cancer.
Biol Pharm Bull. 2019; 42(4):594-600 [PubMed] Related Publications
Sex-related incidence and outcomes were reported in various cancers, including colorectal cancer. 5-Fluorouracil (5-FU) is widely used as an essential chemotherapeutic agent for colorectal cancer. However, sex-based differences in 5-FU toxicity have yet to be reported in human cancer cell lines and xenograft mouse models to date. Here, we investigated, for the first time, sex-based differences in 5-FU toxicity using human colon cancer cell lines, xenograft mouse models, and Korean patients' data. Female-derived colon cancer cell lines exhibited greater 5-FU-induced cytotoxicity than male-derived colon cancer cell lines. We established two xenograft mouse models: one with a male-derived human colon cancer cell line injected into male mice (a male-xenograft model) and another involving a female-derived human colon cancer cell line injected into female mice (a female xenograft model). Treatment with 5-FU inhibited tumor growth and led to hematological toxicity in a female xenograft model more potently than in a male xenograft model. We analyzed the data obtained from Korean patients with colorectal cancer to examine sex differences in adverse drug reactions caused by 5-FU. Korean female patients with colorectal cancer who received 5-FU chemotherapy experienced more frequent adverse drug reactions including alopecia and leukopenia than male patients. Taken together, we demonstrated that female may be associated with increased risk of toxicity to 5-FU treatment in colorectal cancer based on in vitro and in vivo investigations and clinical data analysis. Our study suggests sex as an important clinical factor, which predicts induction of toxicity related to 5-FU treatment.
Ham IH, Oh HJ, Jin H, et al.
Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer.
Mol Cancer. 2019; 18(1):68 [PubMed] Free Access to Full Article Related Publications
Targeting interleukin-6 as a strategy to overcome stroma-induced resistance to chemotherapy in gastric cancer.
Mol Cancer. 2019; 18(1):68 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Although the tumor stroma in solid tumors like gastric cancer (GC) plays a crucial role in chemo-resistance, specific targets to inhibit the interaction between the stromal and cancer cells have not yet been utilized in clinical practice. The present study aims to determine whether cancer-associated fibroblasts (CAFs), a major component of the tumor stroma, confer chemotherapeutic resistance to GC cells, and to discover potential targets to improve chemo-response in GC.
METHODS: To identify CAF-specific proteins and signal transduction pathways affecting chemo-resistance in GC cells, secretome and transcriptome analyses were performed. We evaluated the inhibiting effect of CAF-specific protein in in vivo and in vitro models and investigated the expression of CAF-specific protein in human GC tissues.
RESULTS: Secretome and transcriptome data revealed that interleukin-6 (IL-6) is a CAF-specific secretory protein that protects GC cells via paracrine signaling. Furthermore, CAF-induced activation of the Janus kinase 1-signal transducer and activator of transcription 3 signal transduction pathway confers chemo-resistance in GC cells. CAF-mediated inhibition of chemotherapy-induced apoptosis was abrogated by the anti-IL-6 receptor monoclonal antibody tocilizumab in various experimental models. Clinical data revealed that IL-6 was prominently expressed in the stromal portion of GC tissues, and IL-6 upregulation in GC tissues was correlated with poor responsiveness to chemotherapy.
CONCLUSIONS: Our data provide plausible evidence for crosstalk between GC cells and CAFs, wherein IL-6 is a key contributor to chemoresistance. These findings suggest the potential therapeutic application of IL-6 inhibitors to enhance the responsiveness to chemotherapy in GC.
METHODS: To identify CAF-specific proteins and signal transduction pathways affecting chemo-resistance in GC cells, secretome and transcriptome analyses were performed. We evaluated the inhibiting effect of CAF-specific protein in in vivo and in vitro models and investigated the expression of CAF-specific protein in human GC tissues.
RESULTS: Secretome and transcriptome data revealed that interleukin-6 (IL-6) is a CAF-specific secretory protein that protects GC cells via paracrine signaling. Furthermore, CAF-induced activation of the Janus kinase 1-signal transducer and activator of transcription 3 signal transduction pathway confers chemo-resistance in GC cells. CAF-mediated inhibition of chemotherapy-induced apoptosis was abrogated by the anti-IL-6 receptor monoclonal antibody tocilizumab in various experimental models. Clinical data revealed that IL-6 was prominently expressed in the stromal portion of GC tissues, and IL-6 upregulation in GC tissues was correlated with poor responsiveness to chemotherapy.
CONCLUSIONS: Our data provide plausible evidence for crosstalk between GC cells and CAFs, wherein IL-6 is a key contributor to chemoresistance. These findings suggest the potential therapeutic application of IL-6 inhibitors to enhance the responsiveness to chemotherapy in GC.
Zhan Y, Ma W, Zhang Y, et al.
DNA-Based Nanomedicine with Targeting and Enhancement of Therapeutic Efficacy of Breast Cancer Cells.
ACS Appl Mater Interfaces. 2019; 11(17):15354-15365 [PubMed] Related Publications
DNA-Based Nanomedicine with Targeting and Enhancement of Therapeutic Efficacy of Breast Cancer Cells.
ACS Appl Mater Interfaces. 2019; 11(17):15354-15365 [PubMed] Related Publications
Recently, a DNA tetrahedron has been reported to be a novel nanomedicine and promising drug vector because of its compactness, biocompatibility, biosafety, and editability. Here, we modified the DNA tetrahedron with a DNA aptamer (AS1411) as a DNA-based delivery system, which could bind to nucleolin for its cancer cell selectivity. Nucleolin is a specific biomarker protein overexpressed on membranes of malignant cancer cells and its deregulation is implicated in cell proliferation. The antimetabolite drug 5-fluorouracil (5-FU) is an extensively used anticancer agent; however, its major limitation is the lack of target specificity. Cyanine 5 (Cy5), a fluorescent probe, can be used to label DNA tetrahedron and enhance photostability with minimal effects on its basic functions. In this study, we additionally attached 5-FU to the DNA-based delivery system as a new tumor-targeting nanomedicine (AS1411-T-5-FU) to enhance the therapeutic efficacy and targeting of breast cancer. We examined the difference of the cellular uptake of AS1411-T-5-FU between breast cancer cells and normal breast cells and concluded that AS1411-T-5-FU had a better targeting ability to kill breast cancer cells than 5-FU. We further evaluated the expressions of cell apoptosis-related proteins and genes, which are associated with the mitochondrial apoptotic pathway. Ultimately, our results suggest the potential of DNA tetrahedron in cancer therapies, and we develop a novel approach to endow 5-FU with targeting property.
Yang Y, Bao Y, Yang GK, et al.
MiR-214 sensitizes human colon cancer cells to 5-FU by targeting Hsp27.
Cell Mol Biol Lett. 2019; 24:22 [PubMed] Free Access to Full Article Related Publications
MiR-214 sensitizes human colon cancer cells to 5-FU by targeting Hsp27.
Cell Mol Biol Lett. 2019; 24:22 [PubMed] Free Access to Full Article Related Publications
Overcoming chemorestistance to 5-fluorouracil (5-FU) could offer a new treatment option for highly malignant colon cancer. In our study, differential microRNA expression profiling revealed that miR-214 is downregulated in 5-FU-resistant colon cancer cells compared to normal cells. In vitro, miR-214 could sensitize non-resistant colon cancer cells and 5-FU-resistant colon cancer cellsto 5-FU. Functionally, miR-214 inhibited cell clone formation and cell growth and enhanced 5-FU-inducing cell apoptosis and caspase-3 levels. MiR-214 targeted heat shock protein 27 (Hsp27), as confirmed via dual luciferase reporter assays and western blots. Hsp27 also sensitized HT-29 and LoVo to 5-FU by enhancing cell apoptosis. Overexpression of Hsp27 could block miR-214 with an effect on the sensitivity of colon cancer cells to 5-FU. In conclusion, miR-214 sensitizes colon cancer cells to 5-FU by targeting Hsp27, indicating a significant role for this miRNA in colon cancer chemotherapy.
Iglesias-Puzas A, Batalla A, Suh-Oh HJ, Flórez A
0.5% 5-Fluorouracil/10% Salicylic Acid for the Treatment of Distal Actinic Keratoses Under Daily Practice Conditions
J Drugs Dermatol. 2019; 18(3):285-288 [PubMed] Related Publications
0.5% 5-Fluorouracil/10% Salicylic Acid for the Treatment of Distal Actinic Keratoses Under Daily Practice Conditions
J Drugs Dermatol. 2019; 18(3):285-288 [PubMed] Related Publications
Background: Actinic keratosis (AKs) are sun-induced skin lesions that are at risk to progress to invasive squamous cell carcinoma (SCC). Treatments have shown to be effective on face or balding scalp area but limited data support their efficacy on distal extremities.
Objective: To describe the efficacy of 0.5% 5-fluorouracil/10% salicylic acid (5FU/AS) in the treatment of distally-located AKs in daily clinical practice. Additional objectives were to review tolerance and adherence to this treatment.
Methods: Retrospective review of 23 patients with distal grade II to III AKs who were treated with 5FU/AS under daily practice conditions. Primary endpoint included local skin response according to percentage on AKs reduction at week 20 (8 weeks after ending the treatment).
Results: 75% (30/40) treatment areas showed a percentage reduction in AKs from to 75% to 100% at week 20. Complete response (100% clearance) was recorded in more than half of the cases (53%, 21/40). Good, partial, and low responses were respectively observed in 22% (9/40), 20% (8/40), and 5% (2/40) of patients.
Most adverse events were graded as low, and adherence to treatment was considered correct in 25 patients (63%). In addition, a correct adherence to treatment was significantly related to a better response (P=0.001).
Conclusion: Findings indicate that topical 5FU/AS is an effective treatment for multiple distal AKs, with a proper safety profile.
J Drugs Dermatol. 2019;18(3):285-288.


 Capecitabine
Capecitabine