Found this page useful?
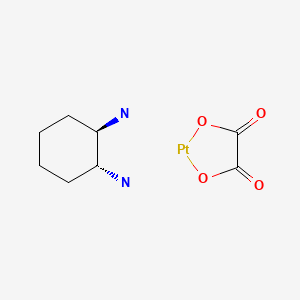
Oxaliplatin
 Web Resources: Oxaliplatin
Web Resources: Oxaliplatin Recent Research Publications
Recent Research PublicationsWeb Resources: Oxaliplatin (6 links)
Cancer Research UK
Macmillan Cancer Support
NHS Evidence
 Oxaliplatin - Substance Summary
Oxaliplatin - Substance Summary
PubChem
Irish Cancer Society
MedlinePlus
Recent Research Publications
Hisaka T, Ishikawa H, Sakai H, et al.
Sinusoidal Obstruction Syndrome and Postoperative Complications Resulting from Preoperative Chemotherapy for Colorectal Cancer Liver Metastasis.
Anticancer Res. 2019; 39(8):4549-4554 [PubMed] Related Publications
Sinusoidal Obstruction Syndrome and Postoperative Complications Resulting from Preoperative Chemotherapy for Colorectal Cancer Liver Metastasis.
Anticancer Res. 2019; 39(8):4549-4554 [PubMed] Related Publications
BACKGROUND/AIM: The aim of this study was to investigate the effects of preoperative chemotherapy on the healthy, metastasis-free part of the liver in colorectal cancer patients with liver metastasis, and the relationship between chemotherapy and postoperative complications.
PATIENTS AND METHODS: Our study included 90 cases of colorectal cancer liver metastasis resected after preoperative chemotherapy. The patients were divided into three groups according to the received chemotherapy regimen: 20 cases received mFOLFOX6, 54 cases a combination of mFOLFOX6 with bevacizumab, and 16 cases a combination of mFOLFOX6 and cetuximab or panitumumab.
RESULTS: The mean numbers of sinusoidal injuries for each chemotherapy type were compared. The group treated with the combination of mFOLFOX6 and bevacizumab showed a lower extent of sinusoidal injury relative to other groups; this intergroup difference became increasingly remarkable as the number of chemotherapy cycles increased. Complications of various extents were found in all three groups, but no significant differences were observed between the three groups.
CONCLUSION: In cases where preoperative chemotherapy was extended over a long period, combined use of bevacizumab was thought to be effective because of stabilization of disturbed liver hemodynamics resulting from sinusoidal injury suppression effects, allowing effective distribution of anti-cancer agents to tumors.
PATIENTS AND METHODS: Our study included 90 cases of colorectal cancer liver metastasis resected after preoperative chemotherapy. The patients were divided into three groups according to the received chemotherapy regimen: 20 cases received mFOLFOX6, 54 cases a combination of mFOLFOX6 with bevacizumab, and 16 cases a combination of mFOLFOX6 and cetuximab or panitumumab.
RESULTS: The mean numbers of sinusoidal injuries for each chemotherapy type were compared. The group treated with the combination of mFOLFOX6 and bevacizumab showed a lower extent of sinusoidal injury relative to other groups; this intergroup difference became increasingly remarkable as the number of chemotherapy cycles increased. Complications of various extents were found in all three groups, but no significant differences were observed between the three groups.
CONCLUSION: In cases where preoperative chemotherapy was extended over a long period, combined use of bevacizumab was thought to be effective because of stabilization of disturbed liver hemodynamics resulting from sinusoidal injury suppression effects, allowing effective distribution of anti-cancer agents to tumors.
Ito Y, Kobuchi S, Takesada W, Takahashi C
Assessment of Oxaliplatin-induced Chronic Neuropathy and Anticancer Efficacy Through Pharmacokinetic and Toxicodynamic Evaluation of a Rat Model of Colorectal Cancer.
Anticancer Res. 2019; 39(8):4207-4213 [PubMed] Related Publications
Assessment of Oxaliplatin-induced Chronic Neuropathy and Anticancer Efficacy Through Pharmacokinetic and Toxicodynamic Evaluation of a Rat Model of Colorectal Cancer.
Anticancer Res. 2019; 39(8):4207-4213 [PubMed] Related Publications
BACKGROUND/AIM: Oxaliplatin-induced chronic neuropathy is a prominent factor for dose reduction and not completing all cycles of chemotherapy for patients with colorectal cancer (CRC). The aim of the study was to investigate the pharmacokinetics and toxicodynamics of oxaliplatin-induced chronic neuropathy in CRC rats to ensure effective management.
MATERIALS AND METHODS: A rat model of CRC was developed using 1,2-Dimethylhydrazine and dextran sulfate. Oxaliplatin (L-OHP) was administered intravenously to CRC rats every week. The pharmacokinetic profiles and tumor distribution of L-OHP and chronic neuropathies were investigated for over four weeks.
RESULTS: The mean values of the area under the concentration-time curve for L-OHP showed a dose-dependent increase. Chronic neuropathy occurred from Day 14 in the 8 mg/kg group and Day 19 in the 3 and 5 mg/kg groups.
CONCLUSION: These results provide preliminary information for the development of a pharmacokinetic and toxicodynamic model of L-OHP for CRC therapy cycles.
MATERIALS AND METHODS: A rat model of CRC was developed using 1,2-Dimethylhydrazine and dextran sulfate. Oxaliplatin (L-OHP) was administered intravenously to CRC rats every week. The pharmacokinetic profiles and tumor distribution of L-OHP and chronic neuropathies were investigated for over four weeks.
RESULTS: The mean values of the area under the concentration-time curve for L-OHP showed a dose-dependent increase. Chronic neuropathy occurred from Day 14 in the 8 mg/kg group and Day 19 in the 3 and 5 mg/kg groups.
CONCLUSION: These results provide preliminary information for the development of a pharmacokinetic and toxicodynamic model of L-OHP for CRC therapy cycles.
Pirozzi A, Riccardi F, Arpino G, et al.
Occurrence of second primary malignancies in patients with neuroendocrine tumors of the digestive tract: A case report.
Medicine (Baltimore). 2019; 98(29):e16508 [PubMed] Related Publications
Occurrence of second primary malignancies in patients with neuroendocrine tumors of the digestive tract: A case report.
Medicine (Baltimore). 2019; 98(29):e16508 [PubMed] Related Publications
RATIONALE: There is an association between the presence of neuroendocrine neoplasms and incremented risk to develop second primary malignancies. This risk is estimated to be 17%. The most common secondary neoplasms were found in the Gastrointestinal and Genitourinary tracts.
PATIENT CONCERNS: A 74-year-old Caucasian patient with melaena came to our observation in June 2015. The Esophago-gastro-duodenoscopy exam found a polypoid formation in the duodenal bulb. Histopathological examination showed a well-differentiated neuroendocrine neoplasm (G1).
DIAGNOSIS: During the follow up for the neuroendocrine neoplasm, a CT scan was performed in August 2016 describing infiltration of the right renal sinus and the third proximal ureter segment with heterogeneous enhancement of vascular structure. An US-guided biopsy was conclusive for a Diffuse Large B Cell Lymphoma. In October 2016, a colonoscopy showed a neoplastic lesion at 20 cm from the anal orifice. The Histology exam was positive for an adenocarcinoma with a desmoplastic stroma infiltration.
INTERVENTIONS: In November 2016, the patient underwent a left hemicolectomy: the pathologic staging described a G2 adenocarcinoma pT3N1b. In May 2018, the Octreotide scan was negative. In the same month, the patient started a treatment based on 6 cycles of Rituximab, Oxaliplatin, and Capecitabine due to the persistence of lymphomatous disease and hepatic metastases. In July 2018, other 3 cycles of the same treatment were scheduled.
OUTCOMES: In January 2019, due to an increase in liver metastases' size, it was decided to start a new regimen for the colon cancer with FOLFIRI+Cetuximab. The patient is still in treatment with this regimen in April 2019.
LESSONS: The risk of a second primary tumor is increased among patients older than 70. Therefore, it is necessary to follow them using total body CT scan and endoscopic techniques of gastrointestinal and genitourinary tracts, not only for the evaluation of the neuroendocrine tumor but also for the higher risk to develop other neoplastic diseases.
PATIENT CONCERNS: A 74-year-old Caucasian patient with melaena came to our observation in June 2015. The Esophago-gastro-duodenoscopy exam found a polypoid formation in the duodenal bulb. Histopathological examination showed a well-differentiated neuroendocrine neoplasm (G1).
DIAGNOSIS: During the follow up for the neuroendocrine neoplasm, a CT scan was performed in August 2016 describing infiltration of the right renal sinus and the third proximal ureter segment with heterogeneous enhancement of vascular structure. An US-guided biopsy was conclusive for a Diffuse Large B Cell Lymphoma. In October 2016, a colonoscopy showed a neoplastic lesion at 20 cm from the anal orifice. The Histology exam was positive for an adenocarcinoma with a desmoplastic stroma infiltration.
INTERVENTIONS: In November 2016, the patient underwent a left hemicolectomy: the pathologic staging described a G2 adenocarcinoma pT3N1b. In May 2018, the Octreotide scan was negative. In the same month, the patient started a treatment based on 6 cycles of Rituximab, Oxaliplatin, and Capecitabine due to the persistence of lymphomatous disease and hepatic metastases. In July 2018, other 3 cycles of the same treatment were scheduled.
OUTCOMES: In January 2019, due to an increase in liver metastases' size, it was decided to start a new regimen for the colon cancer with FOLFIRI+Cetuximab. The patient is still in treatment with this regimen in April 2019.
LESSONS: The risk of a second primary tumor is increased among patients older than 70. Therefore, it is necessary to follow them using total body CT scan and endoscopic techniques of gastrointestinal and genitourinary tracts, not only for the evaluation of the neuroendocrine tumor but also for the higher risk to develop other neoplastic diseases.
Guo Q, Li Q, Wang J, et al.
A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: A preliminary, three-center, clinical trial study.
Medicine (Baltimore). 2019; 98(27):e16234 [PubMed] Free Access to Full Article Related Publications
A comprehensive evaluation of clinical efficacy and safety of celecoxib in combination with chemotherapy in metastatic or postoperative recurrent gastric cancer patients: A preliminary, three-center, clinical trial study.
Medicine (Baltimore). 2019; 98(27):e16234 [PubMed] Free Access to Full Article Related Publications
AIM: To evaluate the efficacy and safety of celecoxib combined with chemotherapy in the treatment of metastatic or postoperative recurrent gastric cancer.
METHODS: This preliminary, three-center, clinical trial study was conducted between September 2010 and December 2016. In the experimental group (n = 100), patients were treated with celecoxib combined with chemotherapy, and chemotherapy alone was used in the control group. Progression-free survival (PFS) was considered as the primary efficacy parameter. Overall survival (OS), remission rate (RR), quality of life (QOL) and drug safety were considered as the secondary efficacy parameters.
RESULTS: The PFS of the experimental group was 6 months, which was not significantly longer than that of the control group (5 months, P = .73). The average OS was not significantly different between the experimental group (12 months) and the control group (10 months, P = .59). The average OS of the COX-2 positive patients in the experimental group was 14 months and it was significantly longer than the 10-month OS in the control group (P = .01). The PFS of the COX-2 positive patients in the experimental group was 7.5 months, significantly longer than the 5-month PFS of patients in the control group (P < .001). No statistical significance was identified in the incidence of nausea, neutropenia, anorexia, peripheral neurotoxicity, diarrhea, vomiting, asthenia and thrombocytopenia. The EORTC QLQ-C30 questionnaire revealed that the overall QOL of the experimental group was significantly higher than that of the control group (P < .05). No statistical significance was found in the scores of functioning scale between the 2 groups. However, the scores of the symptom scale, especially for pain and fatigue in the experimental group was remarkably higher than that in the control group (P < .05). The overall score of EORTC QLQ-STO22 for the experimental group was considerably higher compared to that for the control group (P < .05). No statistical significance was identified in term of the domains of restrictions on feeding, dysphagia, anxiety, reflux, sense of taste, dry mouth, hair loss and body shape between the 2 groups (P > .05 for all mentioned outcomes).
CONCLUSION: Celecoxib combined with chemotherapy offers more clinical benefits for COX-2 positive advanced gastric cancer patients.
METHODS: This preliminary, three-center, clinical trial study was conducted between September 2010 and December 2016. In the experimental group (n = 100), patients were treated with celecoxib combined with chemotherapy, and chemotherapy alone was used in the control group. Progression-free survival (PFS) was considered as the primary efficacy parameter. Overall survival (OS), remission rate (RR), quality of life (QOL) and drug safety were considered as the secondary efficacy parameters.
RESULTS: The PFS of the experimental group was 6 months, which was not significantly longer than that of the control group (5 months, P = .73). The average OS was not significantly different between the experimental group (12 months) and the control group (10 months, P = .59). The average OS of the COX-2 positive patients in the experimental group was 14 months and it was significantly longer than the 10-month OS in the control group (P = .01). The PFS of the COX-2 positive patients in the experimental group was 7.5 months, significantly longer than the 5-month PFS of patients in the control group (P < .001). No statistical significance was identified in the incidence of nausea, neutropenia, anorexia, peripheral neurotoxicity, diarrhea, vomiting, asthenia and thrombocytopenia. The EORTC QLQ-C30 questionnaire revealed that the overall QOL of the experimental group was significantly higher than that of the control group (P < .05). No statistical significance was found in the scores of functioning scale between the 2 groups. However, the scores of the symptom scale, especially for pain and fatigue in the experimental group was remarkably higher than that in the control group (P < .05). The overall score of EORTC QLQ-STO22 for the experimental group was considerably higher compared to that for the control group (P < .05). No statistical significance was identified in term of the domains of restrictions on feeding, dysphagia, anxiety, reflux, sense of taste, dry mouth, hair loss and body shape between the 2 groups (P > .05 for all mentioned outcomes).
CONCLUSION: Celecoxib combined with chemotherapy offers more clinical benefits for COX-2 positive advanced gastric cancer patients.
Ghiringhelli F, Vincent J, Bengrine L, et al.
Hepatic arterial chemotherapy with raltitrexed and oxaliplatin versus standard chemotherapy in unresectable liver metastases from colorectal cancer after conventional chemotherapy failure (HEARTO): a randomized phase-II study.
J Cancer Res Clin Oncol. 2019; 145(9):2357-2363 [PubMed] Related Publications
Hepatic arterial chemotherapy with raltitrexed and oxaliplatin versus standard chemotherapy in unresectable liver metastases from colorectal cancer after conventional chemotherapy failure (HEARTO): a randomized phase-II study.
J Cancer Res Clin Oncol. 2019; 145(9):2357-2363 [PubMed] Related Publications
BACKGROUND: Hepatic arterial infusion (HAI) of chemotherapy could be used in patients with liver-only metastatic colorectal cancer (mCRC) to fight against chemoresistance. We previously reported the efficacy of raltitrexed plus oxaliplatin (HAI) in a retrospective series. We performed a randomized two-stage phase-II study to evaluate the efficacy of HAI of the combination of raltitrexed and oxaliplatin in refractory mCRC with only liver metastases in comparison with standard of care.
PATIENTS AND METHODS: Eligible patients had unresectable mCRC and were refractory or intolerant to fluoropyrimidine, irinotecan, oxaliplatin, anti-VEGF therapy, and anti-EGFR therapy (for tumors with wild-type KRAS). Patients were randomized between HAI raltitrexed (3 mg/m
RESULTS: After inclusion of 27 patients, the trial was terminated due to insufficient accrual. In the experimental arm, 11 and 4 patients experienced grade 3 and 4 toxicities, respectively. The most frequent grade 3-4 toxicities were neutropenia, liver toxicity, and abdominal pain. Median progression-free survival was 6.7 months (95% Confidence Interval; 3.9-7.2) in the HAI group and 2.2 months (95% CI 1.2-4.3) with standard of care [HR 0.32 (95% CI 0.14-0.76), p = 0.01]. Median overall survival did not differ between the two groups, at 11.2 months (95% CI 4.8-17.6) for the HAI group and 11.9 months (95% CI 2.8-14.3) for standard of care [HR 0.86 (95% CI 0.36-2.04), p = 0.73].
CONCLUSION: Although stopped prematurely, this randomized trial provides evidence for the benefit and safety of HAI of a combination of raltitrexed and oxaliplatin in liver-only mCRC with chemoresistant disease.
PATIENTS AND METHODS: Eligible patients had unresectable mCRC and were refractory or intolerant to fluoropyrimidine, irinotecan, oxaliplatin, anti-VEGF therapy, and anti-EGFR therapy (for tumors with wild-type KRAS). Patients were randomized between HAI raltitrexed (3 mg/m
RESULTS: After inclusion of 27 patients, the trial was terminated due to insufficient accrual. In the experimental arm, 11 and 4 patients experienced grade 3 and 4 toxicities, respectively. The most frequent grade 3-4 toxicities were neutropenia, liver toxicity, and abdominal pain. Median progression-free survival was 6.7 months (95% Confidence Interval; 3.9-7.2) in the HAI group and 2.2 months (95% CI 1.2-4.3) with standard of care [HR 0.32 (95% CI 0.14-0.76), p = 0.01]. Median overall survival did not differ between the two groups, at 11.2 months (95% CI 4.8-17.6) for the HAI group and 11.9 months (95% CI 2.8-14.3) for standard of care [HR 0.86 (95% CI 0.36-2.04), p = 0.73].
CONCLUSION: Although stopped prematurely, this randomized trial provides evidence for the benefit and safety of HAI of a combination of raltitrexed and oxaliplatin in liver-only mCRC with chemoresistant disease.
Sugarman R, Patel R, Sharma S, et al.
Pharmacokinetics and pharmacodynamics of new drugs for pancreatic cancer.
Expert Opin Drug Metab Toxicol. 2019; 15(7):541-552 [PubMed] Related Publications
Pharmacokinetics and pharmacodynamics of new drugs for pancreatic cancer.
Expert Opin Drug Metab Toxicol. 2019; 15(7):541-552 [PubMed] Related Publications
Iimura Y, Yasu T, Momo K, et al.
Thiamine deficiency as a possible cofactor causing cognitive dysfunction in a patient with end-stage gastric cancer .
Int J Clin Pharmacol Ther. 2019; 57(8):416-419 [PubMed] Related Publications
Thiamine deficiency as a possible cofactor causing cognitive dysfunction in a patient with end-stage gastric cancer .
Int J Clin Pharmacol Ther. 2019; 57(8):416-419 [PubMed] Related Publications
We describe a case of a patient treated for cognitive dysfunction (CD) with suspected thiamine deficiency (TD). A 74-year-old man with gastric cancer presented with grade 3 diarrhea and grade 1 anorexia. He had been receiving trastuzumab plus tegafur (a chemotherapeutic fluorouracil prodrug), gimeracil, and oteracil (S-1) and oxaliplatin. On admission, cognitive function was assessed with the Hasegawa's Dementia Scale (HDS-R) because he had impaired short-term memory. His thiamine levels increased from 22 to 109 ng/mL after administration of 75 mg of thiamine. Furthermore, the patient's HDS-R score improved from 9 to 22, and cognitive and memory functions improved. TD should be considered in older CD patients receiving oral chemotherapy agents including fluorouracil.
Odin E, Sondén A, Carlsson G, et al.
Folate pathway genes linked to mitochondrial biogenesis and respiration are associated with outcome of patients with stage III colorectal cancer.
Tumour Biol. 2019; 41(6):1010428319846231 [PubMed] Related Publications
Folate pathway genes linked to mitochondrial biogenesis and respiration are associated with outcome of patients with stage III colorectal cancer.
Tumour Biol. 2019; 41(6):1010428319846231 [PubMed] Related Publications
5-fluorouracil in combination with the folate leucovorin is the cornerstone in treatment of colorectal cancer. Transport of leucovorin into cells, and subsequent metabolic action, require expression of several genes. The aim was to analyze if tumoral expression of genes putatively involved in leucovorin transport, polyglutamation, or metabolism was associated with outcome of patients with stage III colorectal cancer treated with adjuvant chemotherapy. A total of 363 stage III colorectal cancer patients who received adjuvant bolus 5-fluorouracil + leucovorin alone, or in combination with oxaliplatin according to Nordic bolus regimes were included. Expression of 11 folate pathway genes was determined in tumors using quantitative real-time polymerase chain reaction and related to disease-free survival. The median follow-up time was 5 years. During follow-up, 114 (31%) patients suffered from recurrent disease. A high tumoral expression of the genes
Panaro F, Kellil T, Vendrell J, et al.
Microvascular invasion is a major prognostic factor after pancreatico-duodenectomy for adenocarcinoma.
J Surg Oncol. 2019; 120(3):483-493 [PubMed] Related Publications
Microvascular invasion is a major prognostic factor after pancreatico-duodenectomy for adenocarcinoma.
J Surg Oncol. 2019; 120(3):483-493 [PubMed] Related Publications
BACKGROUND: Microvascular invasion (MVI) has been proved to be poor prognostic factor in many cancers. To date, only one study published highlights the relationship between this factor and the natural history of pancreatic cancer. The aim of this study was to assess the impact of MVI, on disease-free survival (DFS) and overall survival (OS), after pancreatico-duodenectomy (PD) for pancreatic head adenocarcinoma. Secondarily, we aim to demonstrate that MVI is the most important factor to predict OS after surgery compared with resection margin (RM) and lymph node (LN) status.
MATERIALS AND METHODS: Between January 2015 and December 2017, 158 PD were performed in two hepato-bilio-pancreatic (HBP) centers. Among these, only 79 patients fulfilled the inclusion criteria of the study. Clinical-pathological data and outcomes were retrospectively analyzed from a prospectively maintained database.
RESULTS: Of the 79 patients in the cohort, MVI was identified in 35 (44.3%). In univariate analysis, MVI (P = .012 and P < .0001), RM (P = .023 and P = .021), and LN status (P < .0001 and P = .0001) were significantly associated with DFS and OS. A less than 1 mm margin clearance did not influence relapse (P = .72) or long-term survival (P = .48). LN ratio > 0.226 had a negative impact on OS (P = .044). In multivariate analysis, MVI and RM persisted as independent prognostic factors of DFS (P = .0075 and P = .0098, respectively) and OS (P < .0001 and P = .0194, respectively). Using the likelihood ratio test, MVI was identified as the best fit to predict OS after PD for ductal adenocarcinomas compared with the margin status model (R0 vs R1) (P = .0014).
CONCLUSION: The MVI represents another major prognostic factor determining long-term outcomes.
MATERIALS AND METHODS: Between January 2015 and December 2017, 158 PD were performed in two hepato-bilio-pancreatic (HBP) centers. Among these, only 79 patients fulfilled the inclusion criteria of the study. Clinical-pathological data and outcomes were retrospectively analyzed from a prospectively maintained database.
RESULTS: Of the 79 patients in the cohort, MVI was identified in 35 (44.3%). In univariate analysis, MVI (P = .012 and P < .0001), RM (P = .023 and P = .021), and LN status (P < .0001 and P = .0001) were significantly associated with DFS and OS. A less than 1 mm margin clearance did not influence relapse (P = .72) or long-term survival (P = .48). LN ratio > 0.226 had a negative impact on OS (P = .044). In multivariate analysis, MVI and RM persisted as independent prognostic factors of DFS (P = .0075 and P = .0098, respectively) and OS (P < .0001 and P = .0194, respectively). Using the likelihood ratio test, MVI was identified as the best fit to predict OS after PD for ductal adenocarcinomas compared with the margin status model (R0 vs R1) (P = .0014).
CONCLUSION: The MVI represents another major prognostic factor determining long-term outcomes.
Shen F, Feng L, Zhou J, et al.
Overexpression of CASC11 in ovarian squamous cell carcinoma mediates the development of cancer cell resistance to chemotherapy.
Gene. 2019; 710:363-366 [PubMed] Related Publications
Overexpression of CASC11 in ovarian squamous cell carcinoma mediates the development of cancer cell resistance to chemotherapy.
Gene. 2019; 710:363-366 [PubMed] Related Publications
LncRNA CASC11 promotes gastric cancer and colon cancer. Our study analyzed the role of CASC11 in ovarian squamous cell carcinoma (OSCC). In the present study we showed that plasma CASC11 was upregulated in OSCC, and the upregulation of CASC11 distinguished OSCC patients from control group. Plasma levels of CASC11 were further increased after chemotherapy. Treatment with oxaliplatin, tetraplatin, cisplatin, and carboplatin mediated the upregulation of CASC11 in cells of OSCC cell line. In addition, overexpression of CASC11 led to increased cancer cell viability under oxaliplatin, tetraplatin, cisplatin, and carboplatin treatment, while CASC11 siRNA silencing played an opposite role. Therefore, overexpression of CASC11 in OSCC mediated the development of cancer cell resistance to chemotherapy.
Chen Z, Jiang L
The clinical application of fruquintinib on colorectal cancer.
Expert Rev Clin Pharmacol. 2019; 12(8):713-721 [PubMed] Related Publications
The clinical application of fruquintinib on colorectal cancer.
Expert Rev Clin Pharmacol. 2019; 12(8):713-721 [PubMed] Related Publications
Lin Z, Chen J, Guo Y
Efficacy of XELOX adjuvant chemotherapy for gastric mixed adenoneuroendocrine carcinoma: A case report.
Medicine (Baltimore). 2019; 98(23):e16000 [PubMed] Free Access to Full Article Related Publications
Efficacy of XELOX adjuvant chemotherapy for gastric mixed adenoneuroendocrine carcinoma: A case report.
Medicine (Baltimore). 2019; 98(23):e16000 [PubMed] Free Access to Full Article Related Publications
RATIONALE: Mixed adenoneuroendocrine carcinoma (MANEC) is a rare neoplasm, and consensus on the treatment is unavailable.
PATIENT CONCERN: A 60-year-old Chinese man presented with obstructive symptoms while eating and paroxysmal stomach pain for more than a month.
DIAGNOSIS: MANEC was diagnosed based on clinical manifestations, imaging findings, and pathological examinations.
INTERVENTIONS: The patient underwent radical gastrectomy and received XELOX adjuvant chemotherapy (oxaliplatin 200 mg day 1 + capecitabine 1.5 g twice a day) after surgery.
OUTCOMES: After 4 cycles of XELOX adjuvant chemotherapy were administered, abdominal computerized tomography and liver magnetic resonance showed liver metastasis.
LESSONS: The therapy of gastric MANEC is based on surgical operation, and adjuvant chemotherapy program has an important influence on its prognosis. Therefore, further studying the effectiveness of XELOX adjuvant chemotherapy for gastric MANEC is necessary.
PATIENT CONCERN: A 60-year-old Chinese man presented with obstructive symptoms while eating and paroxysmal stomach pain for more than a month.
DIAGNOSIS: MANEC was diagnosed based on clinical manifestations, imaging findings, and pathological examinations.
INTERVENTIONS: The patient underwent radical gastrectomy and received XELOX adjuvant chemotherapy (oxaliplatin 200 mg day 1 + capecitabine 1.5 g twice a day) after surgery.
OUTCOMES: After 4 cycles of XELOX adjuvant chemotherapy were administered, abdominal computerized tomography and liver magnetic resonance showed liver metastasis.
LESSONS: The therapy of gastric MANEC is based on surgical operation, and adjuvant chemotherapy program has an important influence on its prognosis. Therefore, further studying the effectiveness of XELOX adjuvant chemotherapy for gastric MANEC is necessary.
Xu X, Kong Z, Yi K, et al.
Colonic mucinous adenocarcinoma causing intussusception and distant metastasis: A case report.
Medicine (Baltimore). 2019; 98(21):e15740 [PubMed] Free Access to Full Article Related Publications
Colonic mucinous adenocarcinoma causing intussusception and distant metastasis: A case report.
Medicine (Baltimore). 2019; 98(21):e15740 [PubMed] Free Access to Full Article Related Publications
RATIONALE: Cases of intussusception caused by mucinous carcinoma have been rarely reported, and those caused by colonic mucinous adenocarcinoma (MAC) with distant metastasis were even fewer.
PATIENT CONCERNS: A 60-year-old woman who complained of severe pain around the navel with nausea and vomiting for a week was admitted on November 28, 2017. There were multiple watery stools and abdominal pain was worsened over the prior week.
DIAGNOSIS: She was diagnosed by abdominal computed tomography, current medical history, and abdominal signs. Her initial diagnosis was acute abdomen, intussusceptions, and intestinal obstruction. The final diagnosis was MAC, which was based on postoperative pathology.
INTERVENTIONS: The patient received emergency laparotomy, followed by 5 courses of chemotherapy with oxaliplatin plus capecitabine, and then 6 courses with 5-fluorouracil + oxaliplatin + calcium leucovorin.
OUTCOMES: The patient was in good nutritional condition, and no obvious tumor recurrence or metastasis was found until July 9, 2018.
LESSONS: Even though the prognosis of colonic MAC is poor, being able to receive timely surgical treatment, good nutritional status and reasonable postoperative chemotherapy are the key factors to prolonging patient's survival.
PATIENT CONCERNS: A 60-year-old woman who complained of severe pain around the navel with nausea and vomiting for a week was admitted on November 28, 2017. There were multiple watery stools and abdominal pain was worsened over the prior week.
DIAGNOSIS: She was diagnosed by abdominal computed tomography, current medical history, and abdominal signs. Her initial diagnosis was acute abdomen, intussusceptions, and intestinal obstruction. The final diagnosis was MAC, which was based on postoperative pathology.
INTERVENTIONS: The patient received emergency laparotomy, followed by 5 courses of chemotherapy with oxaliplatin plus capecitabine, and then 6 courses with 5-fluorouracil + oxaliplatin + calcium leucovorin.
OUTCOMES: The patient was in good nutritional condition, and no obvious tumor recurrence or metastasis was found until July 9, 2018.
LESSONS: Even though the prognosis of colonic MAC is poor, being able to receive timely surgical treatment, good nutritional status and reasonable postoperative chemotherapy are the key factors to prolonging patient's survival.
Spartalis C, Schmidt EM, Elmasry M, et al.
In vivo effects of chemotherapy on oncogenic pathways in colorectal cancer.
Cancer Sci. 2019; 110(8):2529-2539 [PubMed] Free Access to Full Article Related Publications
In vivo effects of chemotherapy on oncogenic pathways in colorectal cancer.
Cancer Sci. 2019; 110(8):2529-2539 [PubMed] Free Access to Full Article Related Publications
Patients with advanced colorectal cancer often are treated with systemic cytotoxic therapy using fluorouracil (5-FU), oxaliplatin, irinotecan, and FOLFOX or FOLFIRI combination protocols. Additionally, signaling pathways that are active in colorectal cancer can be therapeutically targeted. Herein, we examined whether chemotherapy impacts on WNT, MAPK and NOTCH signaling pathways in xenograft models of colon cancer. Furthermore, we tested whether combining chemotherapy with MAPK and NOTCH inhibition has superior therapeutic effects. We show that colon cancer cells with high WNT, MAPK and NOTCH activity are variably affected but generally persist in xenograft tumors under different chemotherapeutic regimens, indicating limited effects of cytotoxic therapy on oncogenic signaling pathways. Although these results provided a rationale to additionally target pathway activity, we found no significant increase in therapy response when combining MAPK and NOTCH inhibition with fluorouracil chemotherapy. We attribute this finding to a decrease in tumor cell proliferation upon MAPK and NOTCH inhibition, resulting in reduced effectiveness of cytotoxic treatment. Therapeutic benefits of combining chemotherapy with targeting of oncogenic signaling pathways must therefore be critically evaluated for patients with colorectal cancer.
Alberti P
Platinum-drugs induced peripheral neurotoxicity: clinical course and preclinical evidence.
Expert Opin Drug Metab Toxicol. 2019; 15(6):487-497 [PubMed] Related Publications
Platinum-drugs induced peripheral neurotoxicity: clinical course and preclinical evidence.
Expert Opin Drug Metab Toxicol. 2019; 15(6):487-497 [PubMed] Related Publications
Kim YH, Lee SB, Shim S, et al.
Hyaluronic acid synthase 2 promotes malignant phenotypes of colorectal cancer cells through transforming growth factor beta signaling.
Cancer Sci. 2019; 110(7):2226-2236 [PubMed] Free Access to Full Article Related Publications
Hyaluronic acid synthase 2 promotes malignant phenotypes of colorectal cancer cells through transforming growth factor beta signaling.
Cancer Sci. 2019; 110(7):2226-2236 [PubMed] Free Access to Full Article Related Publications
Hyaluronic acid synthase 2 (HAS2) is suggested to play a critical role in malignancy and is abnormally expressed in many carcinomas. However, its role in colorectal cancer (CRC) malignancy and specific signaling mechanisms remain obscure. Here, we report that HAS2 was markedly increased in both CRC tissue and malignant CRC cell lines. Depletion of HAS2 in HCT116 and DLD1 cells, which express high levels of HAS2, critically increased sensitivity of radiation/oxaliplatin-mediated apoptotic cell death. Moreover, downregulation of HAS2 suppressed migration, invasion and metastasis in nude mice. Conversely, ectopic overexpression of HAS2 in SW480 cells, which express low levels of HAS2, showed the opposite effect. Notably, HAS2 loss- and gain-of-function experiments revealed that it regulates CRC malignancy through TGF-β expression and SMAD2/Snail downstream components. Collectively, our findings suggest that HAS2 contributes to malignant phenotypes of CRC, at least partly, through activation of the TGF-β signaling pathway, and shed light on the novel mechanisms behind the constitutive activation of HAS2 signaling in CRC, thereby highlighting its potential as a therapeutic target.
Xiao C, Qian J, Zheng Y, et al.
A phase II study of biweekly oxaliplatin plus S-1 combination chemotherapy as a first-line treatment for patients with metastatic or advanced gastric cancer in China.
Medicine (Baltimore). 2019; 98(20):e15696 [PubMed] Free Access to Full Article Related Publications
A phase II study of biweekly oxaliplatin plus S-1 combination chemotherapy as a first-line treatment for patients with metastatic or advanced gastric cancer in China.
Medicine (Baltimore). 2019; 98(20):e15696 [PubMed] Free Access to Full Article Related Publications
Oxaliplatin plus S-1 (SOX) was a first-line regimen for advanced gastric cancer. The continuous administration of S-1 for 3 weeks can result in unacceptable gastrointestinal and hematological toxicities. Therefore, an alternative regimen (administration of S-1 for 1-week followed by 1-week rest) is warrant for improved tolerability and noninferiority efficacy. We conducted a study to evaluate the efficacy and safety of biweekly SOX as the first-line chemotherapy in patients with metastatic or advanced gastric cancer in China.Patients with metastatic or previously untreated advanced gastric cancer were enrolled. Oxaliplatin was administered intravenously at a dose of 85 mg/m on day 1, while S-1 was administered orally in doses of 80, 100, or 120 mg/day depending on different body surface areas of <1.25 m, 1.25-1.5 m, or >1.5 m respectively; the total dose of S-1 was administered twice daily on days 1-7 followed by a 7-day rest. This schedule was repeated every 2 weeks until disease progressed or intolerable toxicity occurred.Forty-six patients (M/F = 33/13) received biweekly oxaliplatin and S-1 as first-line chemotherapy. A total of 257 treatment cycles were administered and the median number of cycles administered was 6. Thirty-six patients (78.3%) received second-line chemotherapy. The median progression free survival and median overall survival was 4.4 months (95% CI, 3.37-5.36 months) and 10.3 months (95% CI, 8.88-11.3 months), respectively. The 1-year and 2-year survival rate was 41% and 13%. The objective response rate was 30.43%, and the disease control rate was 76.08%. The observed adverse events of Grade 3/4 included were leukocytopenia (13.04%); anemia (13.04%); neutropenia (15.22%); neurological toxicity (2.17%); diarrhea (2.17%).The biweekly SOX regimen as first-line treatment was active and well tolerated in Chinese patients with metastatic or advanced gastric cancer.
Zhao C, Xie X, Gai DZ, et al.
Interdigitating dendritic cell sarcoma of the spleen with hepatic failure after chemotherapy: A case report.
Medicine (Baltimore). 2019; 98(19):e15535 [PubMed] Free Access to Full Article Related Publications
Interdigitating dendritic cell sarcoma of the spleen with hepatic failure after chemotherapy: A case report.
Medicine (Baltimore). 2019; 98(19):e15535 [PubMed] Free Access to Full Article Related Publications
RATIONALE: Interdigitating dendritic cell sarcoma (IDCS) is an extremely rare disease originating from dendritic cells (DCs). There are few cases report interdigitating dendritic cell sarcoma of spleen along with their pathological characteristics and treatment.
PATIENT CONCERNS: Here we report a case of IDCS in 53-year-old female who presented spleen enlargement and thrombocytopenia.
DIAGNOSES: The patient underwent surgical resection of spleen, and the pathology confirmed IDCS.
INTERVENTIONS: She received surgical resection of spleen and one cycle of chemotherapy (ABVD with ifosfamide and oxaliplatin) after surgery.
OUTCOMES: She died of severe hepatic failure caused by chemotherapy.
DISCUSSION: IDCS is a rare disease with insufficient treatment guidelines. We adopted chemotherapy of ABVD with ifosfamide and oxaliplatin which showed no improvement but led to life-threatening liver damage.
PATIENT CONCERNS: Here we report a case of IDCS in 53-year-old female who presented spleen enlargement and thrombocytopenia.
DIAGNOSES: The patient underwent surgical resection of spleen, and the pathology confirmed IDCS.
INTERVENTIONS: She received surgical resection of spleen and one cycle of chemotherapy (ABVD with ifosfamide and oxaliplatin) after surgery.
OUTCOMES: She died of severe hepatic failure caused by chemotherapy.
DISCUSSION: IDCS is a rare disease with insufficient treatment guidelines. We adopted chemotherapy of ABVD with ifosfamide and oxaliplatin which showed no improvement but led to life-threatening liver damage.
Rovers KP, Bakkers C, Simkens GAAM, et al.
Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6)
BMC Cancer. 2019; 19(1):390 [PubMed] Free Access to Full Article Related Publications
Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6)
BMC Cancer. 2019; 19(1):390 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Upfront cytoreductive surgery with HIPEC (CRS-HIPEC) is the standard treatment for isolated resectable colorectal peritoneal metastases (PM) in the Netherlands. This study investigates whether addition of perioperative systemic therapy to CRS-HIPEC improves oncological outcomes.
METHODS: This open-label, parallel-group, phase II-III, randomised, superiority study is performed in nine Dutch tertiary referral centres. Eligible patients are adults who have a good performance status, histologically or cytologically proven resectable PM of a colorectal adenocarcinoma, no systemic colorectal metastases, no systemic therapy for colorectal cancer within six months prior to enrolment, and no previous CRS-HIPEC. Eligible patients are randomised (1:1) to perioperative systemic therapy and CRS-HIPEC (experimental arm) or upfront CRS-HIPEC alone (control arm) by using central randomisation software with minimisation stratified by a peritoneal cancer index of 0-10 or 11-20, metachronous or synchronous PM, previous systemic therapy for colorectal cancer, and HIPEC with oxaliplatin or mitomycin C. At the treating physician's discretion, perioperative systemic therapy consists of either four 3-weekly neoadjuvant and adjuvant cycles of capecitabine with oxaliplatin (CAPOX), six 2-weekly neoadjuvant and adjuvant cycles of 5-fluorouracil/leucovorin with oxaliplatin (FOLFOX), or six 2-weekly neoadjuvant cycles of 5-fluorouracil/leucovorin with irinotecan (FOLFIRI) followed by four 3-weekly (capecitabine) or six 2-weekly (5-fluorouracil/leucovorin) adjuvant cycles of fluoropyrimidine monotherapy. Bevacizumab is added to the first three (CAPOX) or four (FOLFOX/FOLFIRI) neoadjuvant cycles. The first 80 patients are enrolled in a phase II study to explore the feasibility of accrual and the feasibility, safety, and tolerance of perioperative systemic therapy. If predefined criteria of feasibility and safety are met, the study continues as a phase III study with 3-year overall survival as primary endpoint. A total of 358 patients is needed to detect the hypothesised 15% increase in 3-year overall survival (control arm 50%; experimental arm 65%). Secondary endpoints are surgical characteristics, major postoperative morbidity, progression-free survival, disease-free survival, health-related quality of life, costs, major systemic therapy related toxicity, and objective radiological and histopathological response rates.
DISCUSSION: This is the first randomised study that prospectively compares oncological outcomes of perioperative systemic therapy and CRS-HIPEC with upfront CRS-HIPEC alone for isolated resectable colorectal PM.
TRIAL REGISTRATION: Clinicaltrials.gov/ NCT02758951 , NTR/ NTR6301 , ISRCTN/ ISRCTN15977568 , EudraCT/ 2016-001865-99 .
METHODS: This open-label, parallel-group, phase II-III, randomised, superiority study is performed in nine Dutch tertiary referral centres. Eligible patients are adults who have a good performance status, histologically or cytologically proven resectable PM of a colorectal adenocarcinoma, no systemic colorectal metastases, no systemic therapy for colorectal cancer within six months prior to enrolment, and no previous CRS-HIPEC. Eligible patients are randomised (1:1) to perioperative systemic therapy and CRS-HIPEC (experimental arm) or upfront CRS-HIPEC alone (control arm) by using central randomisation software with minimisation stratified by a peritoneal cancer index of 0-10 or 11-20, metachronous or synchronous PM, previous systemic therapy for colorectal cancer, and HIPEC with oxaliplatin or mitomycin C. At the treating physician's discretion, perioperative systemic therapy consists of either four 3-weekly neoadjuvant and adjuvant cycles of capecitabine with oxaliplatin (CAPOX), six 2-weekly neoadjuvant and adjuvant cycles of 5-fluorouracil/leucovorin with oxaliplatin (FOLFOX), or six 2-weekly neoadjuvant cycles of 5-fluorouracil/leucovorin with irinotecan (FOLFIRI) followed by four 3-weekly (capecitabine) or six 2-weekly (5-fluorouracil/leucovorin) adjuvant cycles of fluoropyrimidine monotherapy. Bevacizumab is added to the first three (CAPOX) or four (FOLFOX/FOLFIRI) neoadjuvant cycles. The first 80 patients are enrolled in a phase II study to explore the feasibility of accrual and the feasibility, safety, and tolerance of perioperative systemic therapy. If predefined criteria of feasibility and safety are met, the study continues as a phase III study with 3-year overall survival as primary endpoint. A total of 358 patients is needed to detect the hypothesised 15% increase in 3-year overall survival (control arm 50%; experimental arm 65%). Secondary endpoints are surgical characteristics, major postoperative morbidity, progression-free survival, disease-free survival, health-related quality of life, costs, major systemic therapy related toxicity, and objective radiological and histopathological response rates.
DISCUSSION: This is the first randomised study that prospectively compares oncological outcomes of perioperative systemic therapy and CRS-HIPEC with upfront CRS-HIPEC alone for isolated resectable colorectal PM.
TRIAL REGISTRATION: Clinicaltrials.gov/ NCT02758951 , NTR/ NTR6301 , ISRCTN/ ISRCTN15977568 , EudraCT/ 2016-001865-99 .
Duan X, Chan C, Han W, et al.
Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors.
Nat Commun. 2019; 10(1):1899 [PubMed] Free Access to Full Article Related Publications
Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors.
Nat Commun. 2019; 10(1):1899 [PubMed] Free Access to Full Article Related Publications
Nanoparticles can potentially stimulate tumour microenvironments to elicit antitumour immunity. Herein, we demonstrate effective immunotherapy of colorectal cancer via systemic delivery of an immunostimulatory chemotherapeutic combination in nanoscale coordination polymer (NCP) core-shell particles. Oxaliplatin and dihydroartemesinin have contrasting physicochemical properties but strong synergy in reactive oxygen species (ROS) generation and anticancer activity. The combined ROS generation is harnessed for immune activation to synergize with an anti-PD-L1 antibody for the treatment of murine colorectal cancer tumours. The favourable biodistribution and tumour uptake of NCPs and the absence of peripheral neuropathy allow for repeated dosing to afford 100% tumour eradication. The involvement of innate and adaptive immune systems elicit strong and long lasting antitumour immunity which prevents tumour formation when cured mice are challenged with cancer cells. The intrinsically biodegradable, well tolerated, and systemically available immunostimulatory NCP promises to enter clinical testing as an immunotherapy against colorectal cancer.
Ulusakarya A, Teyar N, Karaboué A, et al.
Patient-tailored FOLFIRINOX as first line treatment of patients with advanced pancreatic adenocarcinoma.
Medicine (Baltimore). 2019; 98(16):e15341 [PubMed] Free Access to Full Article Related Publications
Patient-tailored FOLFIRINOX as first line treatment of patients with advanced pancreatic adenocarcinoma.
Medicine (Baltimore). 2019; 98(16):e15341 [PubMed] Free Access to Full Article Related Publications
FOLFIRINOX is one of the most effective reference regimens in the 1st line treatment of locally advanced (LA) and metastatic pancreatic cancer (mPC), despite its high toxicity. We evaluated our real-life experience with "patient-tailored intent to treat FOLFIRINOX" in patients with LA or mPC compared to other reports along with the pivotal phase III trial.We analyzed data from all consecutive patients with pancreatic ductal adenocarcinoma treated with dose-modified FOLFIRINOX in 2016 at Paul Brousse University Hospital. Irinotecan was administered whenever initial serum bilirubin was <1.5 × upper limit of normal. Oxaliplatin was stopped for severe sensory neuropathy. Initial dose reductions were made according to patient profile (eg, age, comorbidities) and later due to toxicity. The treatment was continued until surgery or disease progression. Endpoints were time to progression (TTP), overall survival (OS), objective response rate (ORR), and secondary complete resection (R0R1).Thirty-seven patients with unresectable LA or mPC received patient-tailored FOLFIRINOX as 1st line chemotherapy. There were 22 male (59%) and 15 female patients (41%) aged 44 to 81 years with LA (18 patients, 49%) and mPC (19 patients, 51%). They had World Health Organization-performance status of 0 (59%) or 1 (41%). A total of 384 cycles were administered. Median dose intensities (mg/m/w) were 28.9 for oxaliplatin, 56.8 for irinotecan, and 886.2 for 5-fluorouracil. Thirty-four patients were assessed for response; ORR and disease control rates were 47% and 85%, respectively. R0R1 rate was 30%. Median TTP and OS were 9.6 and 14.6 months. LA disease was associated with significantly longer TTP and OS (P < .001).FOLFIRINOX with patient-tailored dose adaptations seems to offer better results in patients with advanced PC. This approach in the neoadjuvant setting results in a macroscopic R0R1 in 61% of patients with initially unresectable disease. It deserves prospective evaluation to further improve outcomes in the management of advanced PC.
Gore M, Hackshaw A, Brady WE, et al.
An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor.
Gynecol Oncol. 2019; 153(3):541-548 [PubMed] Free Access to Full Article Related Publications
An international, phase III randomized trial in patients with mucinous epithelial ovarian cancer (mEOC/GOG 0241) with long-term follow-up: and experience of conducting a clinical trial in a rare gynecological tumor.
Gynecol Oncol. 2019; 153(3):541-548 [PubMed] Free Access to Full Article Related Publications
OBJECTIVES: We evaluated four different treatment regimens for advanced-stage mucinous epithelial ovarian cancer.
METHODS: We conducted a multicenter randomized factorial trial (UK and US). Patients were diagnosed with primary mEOC: FIGO stage II-IV or recurrence after stage I disease. Treatment arms were paclitaxel-carboplatin, oxaliplatin-capecitabine, paclitaxel-carboplatin-bevacizumab, or oxaliplatin-capecitabine-bevacizumab. Chemotherapy was given 3-weekly for 6 cycles, and bevacizumab (3-weekly) was continued as maintenance (for 12 cycles). Endpoints included overall-survival (OS), progression-free survival (PFS), toxicity and quality of life (QoL).
RESULTS: The trial stopped after 50 patients were recruited due to slow accrual. Median follow-up was 59 months. OS hazard ratios (HR) for the two main comparisons were: 0.78 (p = 0.48) for Oxal-Cape vs. Pac-Carbo (each with/without bevacizumab), and 1.04 (p = 0.92) for bevacizumab vs. no bevacizumab. Corresponding PFS HRs were: 0.84 and 0.80. Retrospective central pathology review revealed only 45% (18/40) cases with available material had confirmed primary mEOC. Among these, OS HR for Oxal-Cape vs. Pac-Carbo was 0.36 (p = 0.14); PFS HR = 0.62 (p = 0.40). Grade 3-4 toxicity was seen in 61% Pac-Carbo, 61% Oxal-Cape, 54% Pac-Carbo-Bev, and 85% Oxal-Cape-Bev. QoL was similar between the four arms.
CONCLUSION: mEOC/GOG0241 represents an example of a randomized rare tumor trial. Logistical challenges led to early termination, including difficulties in local histopathological diagnosis and accessing drugs outside their labelled indication. There was misalignment between central funders who support clinical trials in rare cancers and the deprioritisation of such work by those managing and funding research at a local level. Rare cancer trials should include centralised pathology review before treatment. Clinical trial registry number: ISRCTN83438782.
METHODS: We conducted a multicenter randomized factorial trial (UK and US). Patients were diagnosed with primary mEOC: FIGO stage II-IV or recurrence after stage I disease. Treatment arms were paclitaxel-carboplatin, oxaliplatin-capecitabine, paclitaxel-carboplatin-bevacizumab, or oxaliplatin-capecitabine-bevacizumab. Chemotherapy was given 3-weekly for 6 cycles, and bevacizumab (3-weekly) was continued as maintenance (for 12 cycles). Endpoints included overall-survival (OS), progression-free survival (PFS), toxicity and quality of life (QoL).
RESULTS: The trial stopped after 50 patients were recruited due to slow accrual. Median follow-up was 59 months. OS hazard ratios (HR) for the two main comparisons were: 0.78 (p = 0.48) for Oxal-Cape vs. Pac-Carbo (each with/without bevacizumab), and 1.04 (p = 0.92) for bevacizumab vs. no bevacizumab. Corresponding PFS HRs were: 0.84 and 0.80. Retrospective central pathology review revealed only 45% (18/40) cases with available material had confirmed primary mEOC. Among these, OS HR for Oxal-Cape vs. Pac-Carbo was 0.36 (p = 0.14); PFS HR = 0.62 (p = 0.40). Grade 3-4 toxicity was seen in 61% Pac-Carbo, 61% Oxal-Cape, 54% Pac-Carbo-Bev, and 85% Oxal-Cape-Bev. QoL was similar between the four arms.
CONCLUSION: mEOC/GOG0241 represents an example of a randomized rare tumor trial. Logistical challenges led to early termination, including difficulties in local histopathological diagnosis and accessing drugs outside their labelled indication. There was misalignment between central funders who support clinical trials in rare cancers and the deprioritisation of such work by those managing and funding research at a local level. Rare cancer trials should include centralised pathology review before treatment. Clinical trial registry number: ISRCTN83438782.
Lim WQ, Yang G, Phua SZF, et al.
Self-Assembled Oxaliplatin(IV) Prodrug-Porphyrin Conjugate for Combinational Photodynamic Therapy and Chemotherapy.
ACS Appl Mater Interfaces. 2019; 11(18):16391-16401 [PubMed] Related Publications
Self-Assembled Oxaliplatin(IV) Prodrug-Porphyrin Conjugate for Combinational Photodynamic Therapy and Chemotherapy.
ACS Appl Mater Interfaces. 2019; 11(18):16391-16401 [PubMed] Related Publications
Nanomedicine has emerged as a promising strategy for effective cancer treatment. A useful approach is to develop carrier-free nanodrugs via a facile supramolecular self-assembly process. To achieve high therapeutic effect, integrating photodynamic therapy with chemotherapy has been sought after. In this work, we designed a nanocarrier (PEG-Por-CD: oxliPt(IV)-ada) assembled with oxaliplatin prodrug (oxliPt(IV)-ada) and porphyrin photosensitizer (PEG-Por-CD) through host-guest interaction to achieve stimulus-responsive combination therapy. Contributed by excellent spatial control of the binding ratio between host and guest molecules, porphyrin and oxaliplatin were separately modified with β-cyclodextrin and adamantane to prepare the amphiphilic host-guest complex for subsequent self-assembly into therapeutic nanoparticles. The obtained PEG-Por-CD: oxliPt(IV)-ada nanoparticles exhibited good colloidal stability with an average hydrodynamic size of 164 nm while undergoing the disassembly under reductive environment to release active therapeutic species. Confocal imaging demonstrated the ability of PEG-Por-CD: oxliPt(IV)-ada to effectively accumulate in the cells and produce reactive oxygen species in vitro upon 630 nm light irradiation. As compared with the monotherapy, the PEG-Por-CD: oxliPt(IV)-ada nanoparticles exhibited 3-fold enhanced cytotoxicity and 2-fold increase in the apoptosis. In vivo experiments using 4T1 tumor-bearing mice confirmed that the nanoparticles were efficient in suppressing the tumor growth without eliciting systemic toxicity. The present self-delivery nanosystem constructed from the self-assembly approach not only allows precise control over the drug and photosensitizer loading ratio but also eliminates systemic toxicity concern of the drug carriers, providing a solution for further development of combinational cancer treatment.
Larsen FO, Jensen BV, Nørgaard HH, et al.
Intrahepatic Oxaliplatin and Systemic 5-FU +/- Cetuximab in Chemo-Naïve Patients with Liver Metastases from Colorectal Cancer.
Oncology. 2019; 96(6):299-308 [PubMed] Related Publications
Intrahepatic Oxaliplatin and Systemic 5-FU +/- Cetuximab in Chemo-Naïve Patients with Liver Metastases from Colorectal Cancer.
Oncology. 2019; 96(6):299-308 [PubMed] Related Publications
BACKGROUND: In case of response to chemotherapy, unresectable liver metastases from colorectal cancer can be converted to resectable and thereby obtain a chance of cure. The primary aim of this trial was to evaluate the response rate with intrahepatic oxaliplatin in combination with systemic 5-FU +/- cetuximab. Secondary aims were to evaluate the conversion rate from unresectable to resectable liver metastases, median progression-free survival, median overall survival, and toxicity.
METHODS: Forty-five chemo-naïve patients with liver metastases from colorectal cancer were treated in a prospective phase II trial. Calcium folinate and 5-FU were delivered systemically while oxaliplatin was delivered alternating between systemic and intrahepatic administration. When oxaliplatin was delivered intrahepatic-ally, infusion time was reduced to 10 min followed by embolic material. In patients with KRAS wild-type tumors, cetuximab was added.
RESULTS: The treatment was well tolerated and only pain in the liver and a mild increase in liver enzymes were observed after intrahepatic oxaliplatin. The patients obtained a response rate of 82%. Further, 58% converted from having unresectable to resectable liver metastases. The median overall survival and progression-free survival were 38.7 months (95% confidence interval [CI] 33.0-44.3) and 12.9 months (95% CI 10.2-15.6), respectively.
CONCLUSIONS: Intrahepatic infusion of oxaliplatin in 10 min with systemic 5-FU to patients with chemo-naïve colorectal cancer is feasible and with low toxicity. A high response rate and long median overall survival were obtained.
METHODS: Forty-five chemo-naïve patients with liver metastases from colorectal cancer were treated in a prospective phase II trial. Calcium folinate and 5-FU were delivered systemically while oxaliplatin was delivered alternating between systemic and intrahepatic administration. When oxaliplatin was delivered intrahepatic-ally, infusion time was reduced to 10 min followed by embolic material. In patients with KRAS wild-type tumors, cetuximab was added.
RESULTS: The treatment was well tolerated and only pain in the liver and a mild increase in liver enzymes were observed after intrahepatic oxaliplatin. The patients obtained a response rate of 82%. Further, 58% converted from having unresectable to resectable liver metastases. The median overall survival and progression-free survival were 38.7 months (95% confidence interval [CI] 33.0-44.3) and 12.9 months (95% CI 10.2-15.6), respectively.
CONCLUSIONS: Intrahepatic infusion of oxaliplatin in 10 min with systemic 5-FU to patients with chemo-naïve colorectal cancer is feasible and with low toxicity. A high response rate and long median overall survival were obtained.
Al-Batran SE, Homann N, Pauligk C, et al.
Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial.
Lancet. 2019; 393(10184):1948-1957 [PubMed] Related Publications
Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial.
Lancet. 2019; 393(10184):1948-1957 [PubMed] Related Publications
BACKGROUND: Docetaxel-based chemotherapy is effective in metastatic gastric and gastro-oesophageal junction adenocarcinoma. This study reports on the safety and efficacy of the docetaxel-based triplet FLOT (fluorouracil plus leucovorin, oxaliplatin and docetaxel) as a perioperative therapy for patients with locally advanced, resectable tumours.
METHODS: In this controlled, open-label, phase 2/3 trial, we randomly assigned 716 patients with histologically-confirmed advanced clinical stage cT2 or higher or nodal positive stage (cN+), or both, resectable tumours, with no evidence of distant metastases, via central interactive web-based-response system, to receive either three pre-operative and three postoperative 3-week cycles of 50 mg/m
FINDINGS: Between Aug 8, 2010, and Feb 10, 2015, 716 patients were randomly assigned to treatment in 38 German hospitals or with practice-based oncologists. 360 patients were assigned to ECF/ECX and 356 patients to FLOT. Overall survival was increased in the FLOT group compared with the ECF/ECX group (hazard ratio [HR] 0·77; 95% confidence interval [CI; 0.63 to 0·94]; median overall survival, 50 months [38·33 to not reached] vs 35 months [27·35 to 46·26]). The number of patients with related serious adverse events (including those occurring during hospital stay for surgery) was similar in the two groups (96 [27%] in the ECF/ECX group vs 97 [27%] in the FLOT group), as was the number of toxic deaths (two [<1%] in both groups). Hospitalisation for toxicity occurred in 94 patients (26%) in the ECF/ECX group and 89 patients (25%) in the FLOT group.
INTERPRETATION: In locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma, perioperative FLOT improved overall survival compared with perioperative ECF/ECX.
FUNDING: The German Cancer Aid (Deutsche Krebshilfe), Sanofi-Aventis, Chugai, and Stiftung Leben mit Krebs Foundation.
METHODS: In this controlled, open-label, phase 2/3 trial, we randomly assigned 716 patients with histologically-confirmed advanced clinical stage cT2 or higher or nodal positive stage (cN+), or both, resectable tumours, with no evidence of distant metastases, via central interactive web-based-response system, to receive either three pre-operative and three postoperative 3-week cycles of 50 mg/m
FINDINGS: Between Aug 8, 2010, and Feb 10, 2015, 716 patients were randomly assigned to treatment in 38 German hospitals or with practice-based oncologists. 360 patients were assigned to ECF/ECX and 356 patients to FLOT. Overall survival was increased in the FLOT group compared with the ECF/ECX group (hazard ratio [HR] 0·77; 95% confidence interval [CI; 0.63 to 0·94]; median overall survival, 50 months [38·33 to not reached] vs 35 months [27·35 to 46·26]). The number of patients with related serious adverse events (including those occurring during hospital stay for surgery) was similar in the two groups (96 [27%] in the ECF/ECX group vs 97 [27%] in the FLOT group), as was the number of toxic deaths (two [<1%] in both groups). Hospitalisation for toxicity occurred in 94 patients (26%) in the ECF/ECX group and 89 patients (25%) in the FLOT group.
INTERPRETATION: In locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma, perioperative FLOT improved overall survival compared with perioperative ECF/ECX.
FUNDING: The German Cancer Aid (Deutsche Krebshilfe), Sanofi-Aventis, Chugai, and Stiftung Leben mit Krebs Foundation.
Yoshimatsu K, Ishibashi K, Koda K, et al.
A Japanese multicenter phase II study of adjuvant chemotherapy with mFOLFOX6/CAPOX for stage III colon cancer treatment after D2/D3 lymphadenectomy.
Surg Today. 2019; 49(6):498-506 [PubMed] Related Publications
A Japanese multicenter phase II study of adjuvant chemotherapy with mFOLFOX6/CAPOX for stage III colon cancer treatment after D2/D3 lymphadenectomy.
Surg Today. 2019; 49(6):498-506 [PubMed] Related Publications
PURPOSE: A phase II trial was conducted to investigate the benefit of oxaliplatin-based adjuvant chemotherapy in Japanese stage III colon cancer patients.
METHODS: Eligible patients were scheduled to receive 12 cycles of mFOLFOX6 or 8 cycles of CAPOX in adjuvant settings. The primary endpoint was the 3-year disease-free survival (DFS). Cox proportional hazards regression was performed to identify risk factors for a worse DFS.
RESULTS: A total of 130 patients, including 73 patients receiving mFOLFOX6 and 57 patients receiving CAPOX, were enrolled from 16 institutions between April 2010 and April 2014. The 3-year DFS was 82.2%, exceeding the expected primary endpoint of 81.7%. The 3-year DFS tended to be higher in patients receiving mFOLOFOX6 than in those receiving CAPOX (mFOLFOX6, 86.3%; CAPOX, 76.9%; P = 0.06). The 3-year DFS rates did not differ markedly based on the risk stratification (T1/T2/T3 N1 vs. T4 or N2) indicated by the IDEA COLLABORATION study (P = 0.22). In the multivariate analysis, stage IIIC (P = 0.046) and early discontinuation (P < 0.01) were identified as independent significant risk factors for a worse DFS.
CONCLUSION: Our findings represent the first positive results in a Japanese phase II trial of adjuvant chemotherapy with mFOLFOX6/CAPOX. Early discontinuation within 2 months was an independent risk factor for a shorter DFS.
METHODS: Eligible patients were scheduled to receive 12 cycles of mFOLFOX6 or 8 cycles of CAPOX in adjuvant settings. The primary endpoint was the 3-year disease-free survival (DFS). Cox proportional hazards regression was performed to identify risk factors for a worse DFS.
RESULTS: A total of 130 patients, including 73 patients receiving mFOLFOX6 and 57 patients receiving CAPOX, were enrolled from 16 institutions between April 2010 and April 2014. The 3-year DFS was 82.2%, exceeding the expected primary endpoint of 81.7%. The 3-year DFS tended to be higher in patients receiving mFOLOFOX6 than in those receiving CAPOX (mFOLFOX6, 86.3%; CAPOX, 76.9%; P = 0.06). The 3-year DFS rates did not differ markedly based on the risk stratification (T1/T2/T3 N1 vs. T4 or N2) indicated by the IDEA COLLABORATION study (P = 0.22). In the multivariate analysis, stage IIIC (P = 0.046) and early discontinuation (P < 0.01) were identified as independent significant risk factors for a worse DFS.
CONCLUSION: Our findings represent the first positive results in a Japanese phase II trial of adjuvant chemotherapy with mFOLFOX6/CAPOX. Early discontinuation within 2 months was an independent risk factor for a shorter DFS.
Chelakkot PG, Ravind R, Sruthi K, Menon D
Treatment in resectable non-metastatic adenocarcinoma of stomach: Changing paradigms.
Indian J Cancer. 2019 Jan-Mar; 56(1):74-80 [PubMed] Related Publications
Treatment in resectable non-metastatic adenocarcinoma of stomach: Changing paradigms.
Indian J Cancer. 2019 Jan-Mar; 56(1):74-80 [PubMed] Related Publications
Adjuvant treatment in gastric adenocarcinoma has been a challenge for the treating specialists, and despite several trials, a clear consensus is yet to be defined. The higher propensity for lymph nodal involvement and locoregional recurrences led to the hypothesis that locoregional and systemic treatments need to be equally aggressive to achieve better outcomes in the management of gastric adenocarcinoma. Regional, ethnic, and biological differences between the Eastern and Western population are also found to reflect in the tumor behavior and its response to treatment. The MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy), Intergroup 0116, ACTS-GC (Adjuvant Chemotherapy Trial of S-1 for Gastric Cancer), CLASSIC (Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer), ARTIST (Adjuvant Chemoradiation Therapy in Stomach Cancer), and the recently published CRITICS (Chemoradiotherapy after Induction Chemotherapy in Cancer of the Stomach) trials were a few of the randomized controlled trials that tried to give a clearer perspective of this tumor, though it still remains a dilemma. A study incorporating the tumor and demographic factors along with the availability of skilled talent and resources might generate an answer.
Werthmann PG, Kempenich R, Lang-Avérous G, Kienle GS
Long-term survival of a patient with advanced pancreatic cancer under adjunct treatment with
World J Gastroenterol. 2019; 25(12):1524-1530 [PubMed] Free Access to Full Article Related Publications
Long-term survival of a patient with advanced pancreatic cancer under adjunct treatment with
World J Gastroenterol. 2019; 25(12):1524-1530 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Advanced pancreatic cancer (aPC) has a poor prognosis with limited survival benefit from current standard treatment.
CASE SUMMARY: A 59-year-old architect developed epigastric pain. A cystic lesion of the pancreas of 45-mm diameter was detected. In a follow-up magnetic resonance imaging, about one year later, multiple lesions were seen in the corpus and the tail of the pancreas; CA-19-9 was elevated to 58.5 U/mL. A distal pancreatectomy with splenectomy was performed, and a tumor of 7 cm × 5 cm × 3.5 cm was excised. Histologic investigation showed an intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma with invasion of the lymph vessels, perineural invasion, and positive nodes (2/27); surgical margins showed tumor cells, and the tumor was classified as pT3 N1 M0 R1. The patient was treated with radiation of the tumor bed and capecitabine/oxaliplatin followed by gemcitabine and FOLFIRINOX. Seven months after surgery, a liver metastasis was detected and treatment with FOLFIRINOX was started. Four months after detection of the metastasis, the patient opted for additional treatment with VAE. Another month later, the metastasis was treated with radiofrequency ablation (RFA). Eight months later, the hepatic lesion recurred and was again treated with RFA. The continuous VAE treatment was increased in dose, and the patient stayed recurrence-free for the next 39 mo in good health and working full-time (as of the time this case report was written).
CONCLUSION: We present the case of a patient with aPC with R1-resection with development of liver metastasis during the course of treatment who showed an overall survival of 63 mo and a relapse-free survival of 39 mo under increasing VAE therapy. The possible synergistic effect on tumor control of RFA treatment and immune-stimulatory effects of VAE should be further investigated.
CASE SUMMARY: A 59-year-old architect developed epigastric pain. A cystic lesion of the pancreas of 45-mm diameter was detected. In a follow-up magnetic resonance imaging, about one year later, multiple lesions were seen in the corpus and the tail of the pancreas; CA-19-9 was elevated to 58.5 U/mL. A distal pancreatectomy with splenectomy was performed, and a tumor of 7 cm × 5 cm × 3.5 cm was excised. Histologic investigation showed an intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma with invasion of the lymph vessels, perineural invasion, and positive nodes (2/27); surgical margins showed tumor cells, and the tumor was classified as pT3 N1 M0 R1. The patient was treated with radiation of the tumor bed and capecitabine/oxaliplatin followed by gemcitabine and FOLFIRINOX. Seven months after surgery, a liver metastasis was detected and treatment with FOLFIRINOX was started. Four months after detection of the metastasis, the patient opted for additional treatment with VAE. Another month later, the metastasis was treated with radiofrequency ablation (RFA). Eight months later, the hepatic lesion recurred and was again treated with RFA. The continuous VAE treatment was increased in dose, and the patient stayed recurrence-free for the next 39 mo in good health and working full-time (as of the time this case report was written).
CONCLUSION: We present the case of a patient with aPC with R1-resection with development of liver metastasis during the course of treatment who showed an overall survival of 63 mo and a relapse-free survival of 39 mo under increasing VAE therapy. The possible synergistic effect on tumor control of RFA treatment and immune-stimulatory effects of VAE should be further investigated.
Li M, Liu Y, Cui J, et al.
Ulcerative colitis with mucosal lesions in duodenum: Two case reports.
Medicine (Baltimore). 2019; 98(14):e15035 [PubMed] Free Access to Full Article Related Publications
Ulcerative colitis with mucosal lesions in duodenum: Two case reports.
Medicine (Baltimore). 2019; 98(14):e15035 [PubMed] Free Access to Full Article Related Publications
RATIONALE: Ulcerative colitis (UC) is a chronic, nonspecific, inflammatory disease of the colon. Colorectal is the main target organ of UC, while other digestive tract involvement is rare. This report describes 2 rare cases of duodenal mucosa lesions in patients with UC after total colectomy.
PATIENT CONCERNS: In case 1, a patient of 45-year-old with intermittent diarrhea and bloody mucosanguineous feces who was diagnosed as UC, revealed diffuse erosive ulcers in the descending duodenum through gastroscopy after total colectomy. In case 2, a 55-year-old Chinese female with UC, aggravated to colon cancer and received total colectomy. Eighteen months after surgery, the patient was admitted to hospital following upper abdominal pain and acid regurgitation. A gastroscopy found inflammation in the descending part of the duodenum.
DIAGNOSIS: UC, duodenal mucosa lesions INTERVENTIONS:: In case 1, the patient was treated with oral mesalazine (1 g/tid) and hydrocortisone (0.3 g/d) but symptoms did not improve, and the treatment was changed to oral methylprednisolone (0.6 g/d) and a hydrocortisone enema (0.1 g/late). Finally, the patient underwent a total colectomy and ileostomy. In case 2, the patient was treated with sulfasalazine, mesalazine, and intermittent hormone enemas. A total colectomy and ileostomy were performed with the patient after diagnosed as colon cancer. After surgery, the patient received N1-(2 tetrahydrofuryl)-5-fluorouracil (FT-207), 8 g, 300 mg, and 100 mg oxaliplatin chemotherapy, and biologic therapy.
OUTCOMES: In case 1, the patient presented with duodenal necrosis and died of septic shock. In case 2, the patient recovered well without recurrence by taking proton pump inhibitor.
LESSONS: The occurrence of UC related ulcerative gastroduodenal mucosal lesions may be associated with progressing UC or total colitis that does not respond to hormone therapy, leading to requirement of total colectomy.
PATIENT CONCERNS: In case 1, a patient of 45-year-old with intermittent diarrhea and bloody mucosanguineous feces who was diagnosed as UC, revealed diffuse erosive ulcers in the descending duodenum through gastroscopy after total colectomy. In case 2, a 55-year-old Chinese female with UC, aggravated to colon cancer and received total colectomy. Eighteen months after surgery, the patient was admitted to hospital following upper abdominal pain and acid regurgitation. A gastroscopy found inflammation in the descending part of the duodenum.
DIAGNOSIS: UC, duodenal mucosa lesions INTERVENTIONS:: In case 1, the patient was treated with oral mesalazine (1 g/tid) and hydrocortisone (0.3 g/d) but symptoms did not improve, and the treatment was changed to oral methylprednisolone (0.6 g/d) and a hydrocortisone enema (0.1 g/late). Finally, the patient underwent a total colectomy and ileostomy. In case 2, the patient was treated with sulfasalazine, mesalazine, and intermittent hormone enemas. A total colectomy and ileostomy were performed with the patient after diagnosed as colon cancer. After surgery, the patient received N1-(2 tetrahydrofuryl)-5-fluorouracil (FT-207), 8 g, 300 mg, and 100 mg oxaliplatin chemotherapy, and biologic therapy.
OUTCOMES: In case 1, the patient presented with duodenal necrosis and died of septic shock. In case 2, the patient recovered well without recurrence by taking proton pump inhibitor.
LESSONS: The occurrence of UC related ulcerative gastroduodenal mucosal lesions may be associated with progressing UC or total colitis that does not respond to hormone therapy, leading to requirement of total colectomy.
Zhao H, Zhong W, Chen D, Cheng X
Synchronous isolated splenic metastasis from cancer of hepatic flexure of colon: A case report.
Medicine (Baltimore). 2019; 98(14):e15016 [PubMed] Free Access to Full Article Related Publications
Synchronous isolated splenic metastasis from cancer of hepatic flexure of colon: A case report.
Medicine (Baltimore). 2019; 98(14):e15016 [PubMed] Free Access to Full Article Related Publications
RATIONALE: Isolated splenic metastasis from colorectal cancer is very rare, as metastatic colorectal cancer involving the spleen is usually a manifestation of widely disseminated disease. Splenectomy is the best therapeutic option for this entity and probably the only chance for radical cure.
PATIENT CONCERNS: A 73-year-old male presented with abdominal distension and dark red bloody stool of 6-month duration.
DIAGNOSES: Synchronous isolated splenic metastasis from colorectal cancer.
INTERVENTIONS: Based on multidisciplinary team (MDT) mode, the patient underwent the primary hepatic flexure tumor resection due to his poor general condition. One month after surgery the patient began treatment with Xelox (capecitabine 1000 mg/m, oxaliplatin 130 mg/m) every 3 weeks. The patient underwent isolated splenic metastasis resection successfully by laparoscopic after four courses of chemotherapy.
OUTCOMES: The patient's postoperative course was uneventful and he completed four courses of postoperative chemotherapy using the original chemotherapy regimen Xelox (capecitabine 1000 mg/m, oxaliplatin 130 mg/m). The patient was subsequently followed up every 3 months and no signs of recurrence were noted in a recent examination.
LESSONS: To the best of our knowledge, this is the first case report of isolated splenic metastasis from colorectal cancer in China. It is also the first case in which treatment was overseen by an MDT. The possibility of splenic metastasis should be considered in cases in which colorectal cancer is associated with a splenic lesion, despite its rarity. Splenectomy and adjuvant chemotherapy are the optimal therapeutic approaches, as such an approach prolongs survival and palliates the disease.
PATIENT CONCERNS: A 73-year-old male presented with abdominal distension and dark red bloody stool of 6-month duration.
DIAGNOSES: Synchronous isolated splenic metastasis from colorectal cancer.
INTERVENTIONS: Based on multidisciplinary team (MDT) mode, the patient underwent the primary hepatic flexure tumor resection due to his poor general condition. One month after surgery the patient began treatment with Xelox (capecitabine 1000 mg/m, oxaliplatin 130 mg/m) every 3 weeks. The patient underwent isolated splenic metastasis resection successfully by laparoscopic after four courses of chemotherapy.
OUTCOMES: The patient's postoperative course was uneventful and he completed four courses of postoperative chemotherapy using the original chemotherapy regimen Xelox (capecitabine 1000 mg/m, oxaliplatin 130 mg/m). The patient was subsequently followed up every 3 months and no signs of recurrence were noted in a recent examination.
LESSONS: To the best of our knowledge, this is the first case report of isolated splenic metastasis from colorectal cancer in China. It is also the first case in which treatment was overseen by an MDT. The possibility of splenic metastasis should be considered in cases in which colorectal cancer is associated with a splenic lesion, despite its rarity. Splenectomy and adjuvant chemotherapy are the optimal therapeutic approaches, as such an approach prolongs survival and palliates the disease.


 Bevacizumab (Avastin)
Bevacizumab (Avastin)