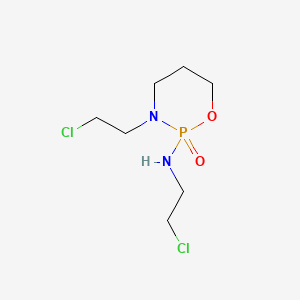
Ifosfamide
"Positional isomer of CYCLOPHOSPHAMIDE which is active as an alkylating agent and an immunosuppressive agent." (MeSH 2013)
Found this page useful?
 Web Resources: Ifosfamide
Web Resources: Ifosfamide Recent Publications: Ifosfamide
Recent Publications: IfosfamideWeb Resources: Ifosfamide (6 links)
Macmillan Cancer Support![]() Content is developed by a team of information development nurses and content editors, and reviewed by health professionals. Further info.
Content is developed by a team of information development nurses and content editors, and reviewed by health professionals. Further info.
Cancer Research UK
NHS Evidence
Includes BNF information
 Ifosfamide - Substance Summary
Ifosfamide - Substance Summary
PubChem
Irish Cancer Society
MedlinePlus.gov
Recent Publications: Ifosfamide
Jiang L, Xu X, Davies H, Shi K
The effect of ifosfamide, epirubicin, and recombinant human endostatin therapy on a cardiac angiosarcoma: A case report.
Medicine (Baltimore). 2019; 98(17):e15290 [PubMed] Related Publications
The effect of ifosfamide, epirubicin, and recombinant human endostatin therapy on a cardiac angiosarcoma: A case report.
Medicine (Baltimore). 2019; 98(17):e15290 [PubMed] Related Publications
RATIONALE: Cardiac angiosarcoma is a rare malignant tumor, for which only surgery has been proven to be effective to date. Currently there are no reports as to whether a postoperative regimen of ifosfamide, epirubicin, and recombinant human endostatin is effective.
PATIENT CONCERN: The patient presented to us with chest pain and dyspnea.
DIAGNOSIS: Enhanced computerized tomography (CT) and positron emission tomography-computerized tomography (PET-CT) suggested pericarditis and an atrial perforation, but malignancy was suspected, so the patient underwent an operation to resect the tumor and repair. Pathology of the tumor reseccted at operation showed the tumor to be an angiosarcoma.
INTERVENTION: After the surgery, the patient was stared on a paclitaxel chemotherapy regimen (135 mg/m once every 3 weeks). However, 2 cycles later, pulmonary and hepatic metastases were found. Chemotherapy was then changed to ifosfamide, epirubicin (ifosfamide 2000 mg/m days 1-3, epirubicin 70 mg/m days 1-2) and recombinant human endostatin (7.5 mg/m days 1-14) in 3 weekly cycles.
OUTCOME: Three cycles later, follow-up showed that chemotherapy had delayed progression of the pulmonary metastases, but that the hepatic node was still growing. The patient has now survived 8 months post surgery and is still on follow-up.
LESSONS: This case shows us that operation on late stage cardiac angiosarcomas can alleviate a patient's symptoms; postoperative paclitaxel monotherapy was insufficient and ifosfamide and epirubicin plus recombinant human endostatin has a limited effect on late stage cardiac angiosarcoma. Studies with a larger sample size are needed for verification of these findings.
PATIENT CONCERN: The patient presented to us with chest pain and dyspnea.
DIAGNOSIS: Enhanced computerized tomography (CT) and positron emission tomography-computerized tomography (PET-CT) suggested pericarditis and an atrial perforation, but malignancy was suspected, so the patient underwent an operation to resect the tumor and repair. Pathology of the tumor reseccted at operation showed the tumor to be an angiosarcoma.
INTERVENTION: After the surgery, the patient was stared on a paclitaxel chemotherapy regimen (135 mg/m once every 3 weeks). However, 2 cycles later, pulmonary and hepatic metastases were found. Chemotherapy was then changed to ifosfamide, epirubicin (ifosfamide 2000 mg/m days 1-3, epirubicin 70 mg/m days 1-2) and recombinant human endostatin (7.5 mg/m days 1-14) in 3 weekly cycles.
OUTCOME: Three cycles later, follow-up showed that chemotherapy had delayed progression of the pulmonary metastases, but that the hepatic node was still growing. The patient has now survived 8 months post surgery and is still on follow-up.
LESSONS: This case shows us that operation on late stage cardiac angiosarcomas can alleviate a patient's symptoms; postoperative paclitaxel monotherapy was insufficient and ifosfamide and epirubicin plus recombinant human endostatin has a limited effect on late stage cardiac angiosarcoma. Studies with a larger sample size are needed for verification of these findings.
Yamaguchi M, Suzuki R, Miyazaki K, et al.
Improved prognosis of extranodal NK/T cell lymphoma, nasal type of nasal origin but not extranasal origin.
Ann Hematol. 2019; 98(7):1647-1655 [PubMed] Related Publications
Improved prognosis of extranodal NK/T cell lymphoma, nasal type of nasal origin but not extranasal origin.
Ann Hematol. 2019; 98(7):1647-1655 [PubMed] Related Publications
Extranodal NK/T cell lymphoma (NKTCL), nasal type (ENKL) that shows no apparent nasal involvement, is termed extranasal NKTCL or non-nasal NKTCL. In this study, we aimed to explore therapeutic approaches and outcomes in patients with extranasal NKTCL in current clinical practice. A data set of patients with newly diagnosed NKTCL who were diagnosed at 31 institutes in Japan between 2000 and 2013 was used for analysis. The patients' fitness for steroid, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy was assessed using the major inclusion criteria of the SMILE phase 2 study. Of 358 patients, 47 (13%) had extranasal NKTCL. The most frequent extranodal sites of involvement in extranasal NKTCL were skin/subcutaneous tissue (n = 18). Six (13%) of the patients with extranasal NKTCL had localized disease and were diagnosed before 2010. With a median follow-up of 5.8 years, the 2-year overall survival (OS) in patients with nasal and extranasal NKTCL was 70% (95% confidence interval [CI], 65-75%) and 34% (95% CI, 21-47%), respectively. OS in patients with nasal NKTCL had a trend toward better according to treatment era (P = 0.063). In contrast, no obvious improvement of OS was observed in extranasal NKTCL (P = 0.43). The major inclusion criteria of the SMILE-P2 were met in 21% (10/47) of patients with extranasal NKTCL and 60% (188/311) of those with nasal NKTCL (P < 0.001). Despite the advent of new treatments for ENKL, OS remains unfavorable in extranasal NKTCL. A more effective therapy is needed for extranasal NKTCL.
Schiavetti A, Pedetti V, Varrasso G, et al.
Long-term renal function and hypertension in adult survivors of childhood sarcoma: Single center experience.
Pediatr Hematol Oncol. 2018; 35(3):167-176 [PubMed] Related Publications
Long-term renal function and hypertension in adult survivors of childhood sarcoma: Single center experience.
Pediatr Hematol Oncol. 2018; 35(3):167-176 [PubMed] Related Publications
AIM: Little data is available on long-term renal impairment in survivors from childhood sarcoma. We investigated the prevalence of renal impairment and hypertension after very long-term follow-up in survivors who reached adulthood after treatment for childhood sarcoma.
METHODS: A cross-sectional single center study was performed. Outcomes included estimating glomerular filtration rate (eGFR), albuminuria, glycosuria, serum phosphate and magnesium, tubular reabsorption phosphate (TRP), chronic kidney disease (CKD) according to the "Kidney Disease: Improving Global Outcomes" (KDIGO) guidelines and blood pressure (BP).
RESULTS: Out of 87 > 5-year sarcoma survivors, 30 adults (10F/20M, median age at diagnosis 9 years, median age at investigation 26 years, median follow-up 16 years, mean 19 years) were identified. Renal impairment was detected in four cases (13.3%); three of these fulfilled the criteria for CKD. Among the adult survivors, a subgroup of 15 cases (50%) had received ifosfamide without confounding factors such as a diagnosis of genito-urinary rhabdomyosarcoma or administration of other potentially nephrotoxic chemotherapy (platinum-based drugs or methotrexate); no renal dysfunction was detected in this subgroup. In the whole cohort of sarcoma survivors, hypertension was diagnosed in four cases (13.3%); BP was significantly correlated with body mass index [p .014].
CONCLUSION: In our series of adult survivors treated for a diagnosis of sarcoma in their childhood, the prevalence of CKD was 10%. We found survivors treated with ifosfamide as the only nephrotoxic agent did not present glomerular or tubular toxicity at long term follow-up, but further studies including a larger number of cases are required to confirm it.
METHODS: A cross-sectional single center study was performed. Outcomes included estimating glomerular filtration rate (eGFR), albuminuria, glycosuria, serum phosphate and magnesium, tubular reabsorption phosphate (TRP), chronic kidney disease (CKD) according to the "Kidney Disease: Improving Global Outcomes" (KDIGO) guidelines and blood pressure (BP).
RESULTS: Out of 87 > 5-year sarcoma survivors, 30 adults (10F/20M, median age at diagnosis 9 years, median age at investigation 26 years, median follow-up 16 years, mean 19 years) were identified. Renal impairment was detected in four cases (13.3%); three of these fulfilled the criteria for CKD. Among the adult survivors, a subgroup of 15 cases (50%) had received ifosfamide without confounding factors such as a diagnosis of genito-urinary rhabdomyosarcoma or administration of other potentially nephrotoxic chemotherapy (platinum-based drugs or methotrexate); no renal dysfunction was detected in this subgroup. In the whole cohort of sarcoma survivors, hypertension was diagnosed in four cases (13.3%); BP was significantly correlated with body mass index [p .014].
CONCLUSION: In our series of adult survivors treated for a diagnosis of sarcoma in their childhood, the prevalence of CKD was 10%. We found survivors treated with ifosfamide as the only nephrotoxic agent did not present glomerular or tubular toxicity at long term follow-up, but further studies including a larger number of cases are required to confirm it.
McKenzie HS, Mead G, Huddart R, et al.
Salvage Chemotherapy With Gemcitabine, Paclitaxel, Ifosfamide, and Cisplatin for Relapsed Germ Cell Cancer.
Clin Genitourin Cancer. 2018; 16(6):458-465.e2 [PubMed] Related Publications
Salvage Chemotherapy With Gemcitabine, Paclitaxel, Ifosfamide, and Cisplatin for Relapsed Germ Cell Cancer.
Clin Genitourin Cancer. 2018; 16(6):458-465.e2 [PubMed] Related Publications
BACKGROUND: Metastatic germ cell tumors remain potentially curable when treated with salvage chemotherapy at first relapse. In the present phase I/II study, we sought to improve on the response rate and duration of the TIP (paclitaxel, ifosfamide, cisplatin) regimen by adding gemcitabine (Gem-TIP).
MATERIALS AND METHODS: Twenty patients were recruited after failure of first-line cisplatin-containing chemotherapy. The primary objectives were to determine the maximum tolerated dose of gemcitabine when combined with TIP and to establish the dose intensity of the TIP drugs in this combination. The secondary objectives were the response rates, failure-free survival, and overall survival.
RESULTS: The maximum tolerated dose of gemcitabine was 1200 mg/m
CONCLUSION: Gemcitabine can be added to TIP chemotherapy at the full dose, with manageable toxicity and no detrimental effect on the dose intensity of the TIP drugs. The response rate and duration were improved compared with those reported from the Medical Research Council TIP trial; further evaluation is warranted.
MATERIALS AND METHODS: Twenty patients were recruited after failure of first-line cisplatin-containing chemotherapy. The primary objectives were to determine the maximum tolerated dose of gemcitabine when combined with TIP and to establish the dose intensity of the TIP drugs in this combination. The secondary objectives were the response rates, failure-free survival, and overall survival.
RESULTS: The maximum tolerated dose of gemcitabine was 1200 mg/m
CONCLUSION: Gemcitabine can be added to TIP chemotherapy at the full dose, with manageable toxicity and no detrimental effect on the dose intensity of the TIP drugs. The response rate and duration were improved compared with those reported from the Medical Research Council TIP trial; further evaluation is warranted.
Savani M, Skubitz KM
Long-term Outcome After Doxorubicin and Ifosfamide Overdose in a Patient With Osteosarcoma and BARD1 Mutation.
J Pediatr Hematol Oncol. 2019; 41(2):e94-e96 [PubMed] Related Publications
Long-term Outcome After Doxorubicin and Ifosfamide Overdose in a Patient With Osteosarcoma and BARD1 Mutation.
J Pediatr Hematol Oncol. 2019; 41(2):e94-e96 [PubMed] Related Publications
Current treatment of high-grade osteosarcoma consists of preoperative chemotherapy, typically using some combination of doxorubicin, cisplatin, ifosfamide, and/or high-dose methotrexate followed by surgical resection. In this report, we present a case of a 21-year-old woman with high-grade osteosarcoma of the chest wall who received 5 times the planned dose of doxorubin and 4 times the planned dose of ifosfamide. She survived this chemotherapy overdose after administration of dimethyl sulfoxide and phenobarbital. Despite the administration of 5 times the proposed dose of doxorubicin, the patient survived without cardiotoxicity, and later delivered a normal baby. Although there are many studies evaluating treatment for chemotherapy regimen-related toxicity, sparse data exist with respect to chemotherapy overdose and the appropriate course of action. This case further confirms the lower cardiotoxicity of continuous intravenous infusion of doxorubicin and provides support for the use of dimethyl sulfoxide in the prevention of toxicity in chemotherapy overdose.
Choi HJ, Paik ES, Choi CH, et al.
Response to Combination Chemotherapy With Paclitaxel/Ifosfamide/Platinum Versus Paclitaxel/Platinum for Patients With Metastatic, Recurrent, or Persistent Carcinoma of the Uterine Cervix: A Retrospective Analysis.
Int J Gynecol Cancer. 2018; 28(7):1333-1341 [PubMed] Related Publications
Response to Combination Chemotherapy With Paclitaxel/Ifosfamide/Platinum Versus Paclitaxel/Platinum for Patients With Metastatic, Recurrent, or Persistent Carcinoma of the Uterine Cervix: A Retrospective Analysis.
Int J Gynecol Cancer. 2018; 28(7):1333-1341 [PubMed] Related Publications
OBJECTIVE: Paclitaxel/ifosfamide/cisplatin triplet has shown a higher response rate than paclitaxel/cisplatin doublet, but the toxicity profile hindered the use of the triplet regimen. In this study, we adjusted the dosage of the triplet regimen and introduced carboplatin in cisplatin-intolerable patients. We tested the efficacy and toxicity of the modified triplet regimen in patients with recurrent or persistent cervical cancer.
MATERIALS AND METHODS: We retrospectively reviewed the medical records of patients with recurrent or persistent cervical cancer who were treated between 2003 and 2015 at Samsung Medical Center. Response rate, progression-free survival (PFS), overall survival (OS), and toxicity of paclitaxel/ifosfamide/platinum (TIP) and paclitaxel/platinum (TP) were compared.
RESULTS: The overall response rate of TIP was significantly higher than that of TP (52.7% vs 36.4%, P = 0.031). In the TP group, response rate was higher in patients with progression-free interval longer than 12 months (P = 0.028) and those with squamous cell histology (P = 0.028). In TIP group, patients with older than 50 years (P = 0.017), progression-free interval longer than 12 months (P = 0.046), and squamous cell carcinoma histology (P < 0.001) showed higher response rates; but TIP showed higher response on all occasions. Median OS and median PFS were similar for TP and TIP (OS, 22.43 months vs 18.5 months, P = 0.44; PFS, 6.37 months vs 8.3 months, P = 0.48).
CONCLUSIONS: Paclitaxel/ifosfamide/platinum showed a higher response rate than TP in patients with recurrent cervical cancer without an increase in severe complications. Considering the high response rate, TIP may be an option for persistent or recurrent cervical cancer.
MATERIALS AND METHODS: We retrospectively reviewed the medical records of patients with recurrent or persistent cervical cancer who were treated between 2003 and 2015 at Samsung Medical Center. Response rate, progression-free survival (PFS), overall survival (OS), and toxicity of paclitaxel/ifosfamide/platinum (TIP) and paclitaxel/platinum (TP) were compared.
RESULTS: The overall response rate of TIP was significantly higher than that of TP (52.7% vs 36.4%, P = 0.031). In the TP group, response rate was higher in patients with progression-free interval longer than 12 months (P = 0.028) and those with squamous cell histology (P = 0.028). In TIP group, patients with older than 50 years (P = 0.017), progression-free interval longer than 12 months (P = 0.046), and squamous cell carcinoma histology (P < 0.001) showed higher response rates; but TIP showed higher response on all occasions. Median OS and median PFS were similar for TP and TIP (OS, 22.43 months vs 18.5 months, P = 0.44; PFS, 6.37 months vs 8.3 months, P = 0.48).
CONCLUSIONS: Paclitaxel/ifosfamide/platinum showed a higher response rate than TP in patients with recurrent cervical cancer without an increase in severe complications. Considering the high response rate, TIP may be an option for persistent or recurrent cervical cancer.
Lee J, Jung HA, Kim Y, et al.
Efficacy of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in patients with advanced pulmonary pleomorphic carcinoma.
Lung Cancer. 2018; 122:160-164 [PubMed] Related Publications
Efficacy of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in patients with advanced pulmonary pleomorphic carcinoma.
Lung Cancer. 2018; 122:160-164 [PubMed] Related Publications
OBJECTIVES: Pulmonary pleomorphic carcinoma (PC) is a rare type of lung tumor with a dismal prognosis. There is no consensus on a chemotherapy regimen for PC, and conventional platinum-based chemotherapy has been associated with disappointing response rates and PFS. In searches for a new regimen, the sarcomatoid (spindle or giant cell) component has been assumed to be susceptible to chemotherapy used for soft tissue sarcoma.
MATERIALS AND METHODS: The medical records of 17 patients who received mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) for advanced PC between January 2010 and February 2017 were retrospectively analyzed for clinicopathological features and outcomes.
RESULTS AND CONCLUSION: The median age was 59 years. Sixteen patients were male, and only one patient had never smoked. Six patients achieved partial response to MAID, leading to an objective response rate of 35%. The median PFS was 2.8 months, and the median OS was 8.7 months. Hematologic toxicity-related adverse events were the most frequent, which comprised grade 3-4 anemia in 35% of patients, neutropenia in 47%, thrombocytopenia in 24%, and febrile neutropenia in 29%. No febrile neutropenia was reported in patients who received 5-day granulocyte-colony stimulating factor (G-CSF) prophylaxis. Most adverse events resolved without complications, except for one death due to sepsis. MAID is an effective, and possibly important, regimen for PC. MAID could be more safely used in clinical practice with appropriate dose modifications and G-CSF primary prophylaxis according to patients' status.
MATERIALS AND METHODS: The medical records of 17 patients who received mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) for advanced PC between January 2010 and February 2017 were retrospectively analyzed for clinicopathological features and outcomes.
RESULTS AND CONCLUSION: The median age was 59 years. Sixteen patients were male, and only one patient had never smoked. Six patients achieved partial response to MAID, leading to an objective response rate of 35%. The median PFS was 2.8 months, and the median OS was 8.7 months. Hematologic toxicity-related adverse events were the most frequent, which comprised grade 3-4 anemia in 35% of patients, neutropenia in 47%, thrombocytopenia in 24%, and febrile neutropenia in 29%. No febrile neutropenia was reported in patients who received 5-day granulocyte-colony stimulating factor (G-CSF) prophylaxis. Most adverse events resolved without complications, except for one death due to sepsis. MAID is an effective, and possibly important, regimen for PC. MAID could be more safely used in clinical practice with appropriate dose modifications and G-CSF primary prophylaxis according to patients' status.
Bisogno G, Jenney M, Bergeron C, et al.
Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): a multicentre, open-label, randomised controlled, phase 3 trial.
Lancet Oncol. 2018; 19(8):1061-1071 [PubMed] Related Publications
Addition of dose-intensified doxorubicin to standard chemotherapy for rhabdomyosarcoma (EpSSG RMS 2005): a multicentre, open-label, randomised controlled, phase 3 trial.
Lancet Oncol. 2018; 19(8):1061-1071 [PubMed] Related Publications
BACKGROUND: Rhabdomyosarcoma is an aggressive tumour that can develop in almost any part of the body. Doxorubicin is an effective drug against rhabdomyosarcoma, but its role in combination with an established multidrug regimen remains controversial. Therefore, we aimed to evaluate the possible benefit of early dose intensification with doxorubicin in patients with non-metastatic rhabdomyosarcoma.
METHODS: We did a multicentre, open-label, randomised controlled, phase 3 trial involving 108 hospitals from 14 countries. We included patients older than 6 months but younger than 21 years with a pathologically proven diagnosis of rhabdomyosarcoma. We assigned each patient to a specific subgroup according to the EpSSG stratification system. Those with embryonal rhabdomyosarcoma incompletely resected and localised at unfavourable sites with or without nodal involvement, or those with alveolar rhabdomyosarcoma without nodal involvement were considered at high risk of relapse. These high-risk patients were randomly assigned (1:1) to receive either nine cycles of IVA (ifosfamide 3 g/m
FINDINGS: Between Oct 1, 2005, and Dec 16, 2013, 484 patients were randomly assigned to receive each chemotherapy regimen (242 in the IVA group and 242 in the IVA plus doxorubicin group). Median follow-up was 63·9 months (IQR 44·6-78·9). The 3-year event-free survival was 67·5% (95% CI 61·2-73·1) in the IVA plus doxorubicin group and 63·3% (56·8-69·0) in the IVA group (hazard ratio 0·87, 95% CI 0·65-1·16; p=0·33). Grade 3-4 leucopenia (232 [93%] of 249 patients in the IVA plus doxorubicin group vs 194 [85%] of 227 in the IVA group; p=0·0061), anaemia (195 [78%] vs 111 [49%]; p<0·0001), thrombocytopenia (168 [67%] vs 59 [26%]; p<0.0001), and gastrointestinal adverse events (78 [31%] vs 19 [8%]; p<0·0001) were significantly more common in the IVA plus doxorubicin group than in the IVA group. Grade 3-5 infections (198 [79%] vs 128 [56%]; p<0·0001) were also significantly more common in the IVA plus doxorubicin group than in the IVA group, in which one patient had grade 5 infection. Two treatment-related deaths were reported (one patient developed septic shock and one affected by Goldenhar syndrome developed intractable seizures) in the IVA plus doxorubicin group, both occurring after the first cycle of treatment, and none were reported in the IVA group.
INTERPRETATIONS: The addition of dose-intensified doxorubicin to standard IVA chemotherapy did not show a significant improvement in the outcome of patients with high-risk non-metastatic rhabdomyosarcoma. Therefore, the IVA chemotherapy regimen should remain the standard of care for patients with localised rhabdomyosarcoma in Europe.
FUNDING: Fondazione Città della Speranza, Italy, and the Association Léon Berard Enfant Cancéreux, France.
METHODS: We did a multicentre, open-label, randomised controlled, phase 3 trial involving 108 hospitals from 14 countries. We included patients older than 6 months but younger than 21 years with a pathologically proven diagnosis of rhabdomyosarcoma. We assigned each patient to a specific subgroup according to the EpSSG stratification system. Those with embryonal rhabdomyosarcoma incompletely resected and localised at unfavourable sites with or without nodal involvement, or those with alveolar rhabdomyosarcoma without nodal involvement were considered at high risk of relapse. These high-risk patients were randomly assigned (1:1) to receive either nine cycles of IVA (ifosfamide 3 g/m
FINDINGS: Between Oct 1, 2005, and Dec 16, 2013, 484 patients were randomly assigned to receive each chemotherapy regimen (242 in the IVA group and 242 in the IVA plus doxorubicin group). Median follow-up was 63·9 months (IQR 44·6-78·9). The 3-year event-free survival was 67·5% (95% CI 61·2-73·1) in the IVA plus doxorubicin group and 63·3% (56·8-69·0) in the IVA group (hazard ratio 0·87, 95% CI 0·65-1·16; p=0·33). Grade 3-4 leucopenia (232 [93%] of 249 patients in the IVA plus doxorubicin group vs 194 [85%] of 227 in the IVA group; p=0·0061), anaemia (195 [78%] vs 111 [49%]; p<0·0001), thrombocytopenia (168 [67%] vs 59 [26%]; p<0.0001), and gastrointestinal adverse events (78 [31%] vs 19 [8%]; p<0·0001) were significantly more common in the IVA plus doxorubicin group than in the IVA group. Grade 3-5 infections (198 [79%] vs 128 [56%]; p<0·0001) were also significantly more common in the IVA plus doxorubicin group than in the IVA group, in which one patient had grade 5 infection. Two treatment-related deaths were reported (one patient developed septic shock and one affected by Goldenhar syndrome developed intractable seizures) in the IVA plus doxorubicin group, both occurring after the first cycle of treatment, and none were reported in the IVA group.
INTERPRETATIONS: The addition of dose-intensified doxorubicin to standard IVA chemotherapy did not show a significant improvement in the outcome of patients with high-risk non-metastatic rhabdomyosarcoma. Therefore, the IVA chemotherapy regimen should remain the standard of care for patients with localised rhabdomyosarcoma in Europe.
FUNDING: Fondazione Città della Speranza, Italy, and the Association Léon Berard Enfant Cancéreux, France.
Pashankar F, Frazier AL, Krailo M, et al.
Treatment of refractory germ cell tumors in children with paclitaxel, ifosfamide, and carboplatin: A report from the Children's Oncology Group AGCT0521 study.
Pediatr Blood Cancer. 2018; 65(8):e27111 [PubMed] Free Access to Full Article Related Publications
Treatment of refractory germ cell tumors in children with paclitaxel, ifosfamide, and carboplatin: A report from the Children's Oncology Group AGCT0521 study.
Pediatr Blood Cancer. 2018; 65(8):e27111 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Paclitaxel, ifosfamide, cisplatin (TIP) is commonly used as salvage for malignant germ cell tumors (MGCT) in adults; however, additional administration of cisplatin at a young age could cause significant short- and long-term toxicities in a group of patients with high expected salvage. Because carboplatin has been shown to be effective in pediatric MGCT with less toxicity, the TIP regimen was modified by substituting carboplatin for cisplatin.
METHODS: The Children's Oncology Group conducted a phase II trial between November 2007 and June 2011 evaluating "TIC" (paclitaxel 135 mg/m
RESULTS: Twenty patients (12 male, median age 13.5 years) were enrolled. Seventeen patients had tumor markers ≥10 times above normal. After two cycles, by RECIST criteria, 8 patients achieved a partial response (response rate 40%), 10 had stable disease, and 2 had progressive disease. A ≥ 1 log reduction was achieved in 10/17 patients (58.8%) with elevated markers. By study defined criteria, combining response by RECIST and marker decline, the response rate was 44%.
CONCLUSION: TIC is active in relapsed pediatric MGCT and should be considered for salvage therapy in children. In adolescents and older adults with relapse MGCT, TIP or high-dose chemotherapy with stem cell remain the standard therapy.
METHODS: The Children's Oncology Group conducted a phase II trial between November 2007 and June 2011 evaluating "TIC" (paclitaxel 135 mg/m
RESULTS: Twenty patients (12 male, median age 13.5 years) were enrolled. Seventeen patients had tumor markers ≥10 times above normal. After two cycles, by RECIST criteria, 8 patients achieved a partial response (response rate 40%), 10 had stable disease, and 2 had progressive disease. A ≥ 1 log reduction was achieved in 10/17 patients (58.8%) with elevated markers. By study defined criteria, combining response by RECIST and marker decline, the response rate was 44%.
CONCLUSION: TIC is active in relapsed pediatric MGCT and should be considered for salvage therapy in children. In adolescents and older adults with relapse MGCT, TIP or high-dose chemotherapy with stem cell remain the standard therapy.
Pennington JD, Eilber FC, Eilber FR, et al.
Long-term Outcomes With Ifosfamide-based Hypofractionated Preoperative Chemoradiotherapy for Extremity Soft Tissue Sarcomas.
Am J Clin Oncol. 2018; 41(12):1154-1161 [PubMed] Related Publications
Long-term Outcomes With Ifosfamide-based Hypofractionated Preoperative Chemoradiotherapy for Extremity Soft Tissue Sarcomas.
Am J Clin Oncol. 2018; 41(12):1154-1161 [PubMed] Related Publications
OBJECTIVES: The objective of this study was to analyze outcomes for patients with soft tissue sarcoma of the extremities using neoadjuvant ifosfamide-based chemotherapy and hypofractionated reduced dose radiotherapy, followed by limb-sparing surgery.
MATERIALS AND METHODS: An Institutional Review Board (IRB)-approved retrospective review of patients treated at a single institution between 1990 and 2013 was performed. In total, 116 patients were identified who received neoadjuvant ifosfamide-based chemotherapy and 28 Gy in 8 fractions of preoperative radiation (equivalent dose in 2 Gray fractions, 31.5 Gy [α/β 10] 36.4 Gy [α/β 3]) followed by limb-sparing surgery. Local recurrence (LR), distant failure (DF), and overall survival (OS) were calculated. Univariate and multivariate analysis for LR, DF, and OS were performed using Cox analysis. Statistical significance was set at a P<0.05.
RESULTS: Median follow-up was 5.9 years (range, 0.3 to 24 y). Actuarial LR at 3/6 years was 11%/17%, DF at 3/6 years was 25%/35%, and OS at 3/6 years was 82%/67%. On multivariate analysis, only a positive surgical margin was significantly correlated with worse local control (P=0.005; hazard ratio [HR], 18.33; 95% confidence interval (CI), 2.41-139.34). Age over 60 years (P=0.03; HR, 2.34; 95% CI, 1.10-4.98) and tumor size over 10 cm compared with tumor size ≤5 cm (P=0.03; HR, 3.32; 95% CI, 1.15-9.61) were associated with worse OS.
CONCLUSIONS: Soft tissue extremity sarcoma patients treated using reduced dose hypofractionated preoperative radiotherapy in combination with ifosfamide-based chemotherapy shows acceptable local control and warrants further investigation.
MATERIALS AND METHODS: An Institutional Review Board (IRB)-approved retrospective review of patients treated at a single institution between 1990 and 2013 was performed. In total, 116 patients were identified who received neoadjuvant ifosfamide-based chemotherapy and 28 Gy in 8 fractions of preoperative radiation (equivalent dose in 2 Gray fractions, 31.5 Gy [α/β 10] 36.4 Gy [α/β 3]) followed by limb-sparing surgery. Local recurrence (LR), distant failure (DF), and overall survival (OS) were calculated. Univariate and multivariate analysis for LR, DF, and OS were performed using Cox analysis. Statistical significance was set at a P<0.05.
RESULTS: Median follow-up was 5.9 years (range, 0.3 to 24 y). Actuarial LR at 3/6 years was 11%/17%, DF at 3/6 years was 25%/35%, and OS at 3/6 years was 82%/67%. On multivariate analysis, only a positive surgical margin was significantly correlated with worse local control (P=0.005; hazard ratio [HR], 18.33; 95% confidence interval (CI), 2.41-139.34). Age over 60 years (P=0.03; HR, 2.34; 95% CI, 1.10-4.98) and tumor size over 10 cm compared with tumor size ≤5 cm (P=0.03; HR, 3.32; 95% CI, 1.15-9.61) were associated with worse OS.
CONCLUSIONS: Soft tissue extremity sarcoma patients treated using reduced dose hypofractionated preoperative radiotherapy in combination with ifosfamide-based chemotherapy shows acceptable local control and warrants further investigation.
Akin S, Dizdar O, Karakas Y, et al.
Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcoma.
Curr Probl Cancer. 2018 May - Jun; 42(3):344-349 [PubMed] Related Publications
Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcoma.
Curr Probl Cancer. 2018 May - Jun; 42(3):344-349 [PubMed] Related Publications
Leiomyosarcomas (LMS) are rare tumors with poor prognosis owing to the high rate of recurrent and metastatic disease. The combination of doxorubicin (Adriamycin) plus ifosfamide and mesna (AIM) results in moderate response rates of 10%-30%. The aim of this study was to assess the efficacy of the AIM regimen along with multimodality treatment including surgery and radiotherapy in patients with LMS. The clinicopathologic characteristics and outcomes of 51 patients with recurrent or metastatic LMS diagnosed between 2000 and 2014 who received the AIM regimen were analyzed retrospectively. Treatment consisted of ifosfamide 2500mg/m² on days 1-3 (with mesna 2500mg/m² days 1-3, 4-hour i.v. infusion), and doxorubicin 60mg/m² on day 1 (2-hour i.v. infusion), which was repeated every 21 days. The mean age of the patients at diagnosis was 48.9 ± 11.2 years. A total of 42 patients were females (82.4%). The primary tumor site was the uterus in 30 (58.8%) patients. The most common metastatic sites were lung and liver. The median follow-up was 27.9 months (min: 4.3 max: 164.8). The median progression-free survival was 6.7 months (95% CI: 4.1-9.2). The median overall survival (OS) was 24.6 months (95% CI: 16.2-33.0). The overall response rate was 12% (6/51 pts). Response rates were higher in patients with uterine LMS (17%) compared with those with nonuterine LMS (5%); however, the OS times were similar. Surgical intervention for local or distant recurrence was associated with improved median OS (41 vs 16.6 months, P < 0.001). Myelosuppression was the major toxicity of this combination. In our study, the AIM regimen was effective in patients with LMS. Resection of local or distant recurrence was found to improve survival in our study.
García Del Muro X, Maurel J, Martínez Trufero J, et al.
Phase II trial of ifosfamide in combination with the VEGFR inhibitor sorafenib in advanced soft tissue sarcoma: a Spanish group for research on sarcomas (GEIS) study.
Invest New Drugs. 2018; 36(3):468-475 [PubMed] Related Publications
Phase II trial of ifosfamide in combination with the VEGFR inhibitor sorafenib in advanced soft tissue sarcoma: a Spanish group for research on sarcomas (GEIS) study.
Invest New Drugs. 2018; 36(3):468-475 [PubMed] Related Publications
Background Sorafenib is a potent targeted-therapy that blockades angiogenesis and has demonstrated activity against some sarcoma subtypes. Preclinical studies suggested that treatment with sorafenib plus cytotoxic agents could result in additive efficacy. Methods Patients with advanced soft tissue sarcoma, with or without anthracycline pretreatment were included. Patients received oral sorafenib 400 mg twice daily starting on Day +2, ifosfamide 2.0 g/m2 iv infusion lasting 4 h on days 1, 2 and 3 with concurrent mesna 400 mg/m
Aoyama T, Imataki O, Arai H, et al.
Comparison of Nutrition-Related Adverse Events and Clinical Outcomes Between ICE (Ifosfamide, Carboplatin, and Etoposide) and MCEC (Ranimustine, Carboplatin, Etoposide, and Cyclophosphamide) Therapies as Pretreatment for Autologous Peripheral Blood Stem Cell Transplantation in Patients with Malignant Lymphoma.
Med Sci Monit Basic Res. 2018; 24:31-39 [PubMed] Free Access to Full Article Related Publications
Comparison of Nutrition-Related Adverse Events and Clinical Outcomes Between ICE (Ifosfamide, Carboplatin, and Etoposide) and MCEC (Ranimustine, Carboplatin, Etoposide, and Cyclophosphamide) Therapies as Pretreatment for Autologous Peripheral Blood Stem Cell Transplantation in Patients with Malignant Lymphoma.
Med Sci Monit Basic Res. 2018; 24:31-39 [PubMed] Free Access to Full Article Related Publications
BACKGROUND The aim of this study was to compare nutrition-related adverse events and clinical outcomes of ifosfamide, carboplatin, and etoposide regimen (ICE therapy) and ranimustine, carboplatin, etoposide, and cyclophosphamide regimen (MCEC therapy) instituted as pretreatment for autologous peripheral blood stem cell transplantation. MATERIAL AND METHODS We enrolled patients who underwent autologous peripheral blood stem cell transplantation between 2007 and 2012. Outcomes were compared between ICE therapy (n=14) and MCEC therapy (n=14) in relation to nutrient balance, engraftment day, and length of hospital stay. In both groups, we compared the timing of nutrition-related adverse events with oral caloric intake, analyzed the correlation between length of hospital stay and duration of parenteral nutrition, and investigated the association between oral caloric intake and the proportion of parenteral nutrition energy in total calorie supply. Five-year survival was compared between the groups. RESULTS Compared with the MCEC group, the ICE group showed significant improvement in oral caloric intake, length of hospital stay, and timing of nutrition-related adverse events and oral calorie intake, but a delay in engraftment. Both groups showed a correlation between duration of parenteral nutrition and length of hospital stay (P=0.0001) and between oral caloric intake (P=0.0017) and parenteral nutrition energy sufficiency rate (r=-0.73, P=0.003; r=-0.76, P=0.002). Five-year survival was not significantly different between the groups (P=0.1355). CONCLUSIONS Our findings suggest that compared with MCEC therapy, ICE therapy improves nutrition-related adverse events and reduces hospital stay, conserving medical resources, with no significant improvement in long-term survival. The nutritional pathway may serve as a tool for objective evaluation of pretreatment for autologous peripheral blood stem cell transplantation.
Lu E, Perlewitz KS, Hayden JB, et al.
Epirubicin and Ifosfamide with Preoperative Radiation for High-Risk Soft Tissue Sarcomas.
Ann Surg Oncol. 2018; 25(4):920-927 [PubMed] Related Publications
Epirubicin and Ifosfamide with Preoperative Radiation for High-Risk Soft Tissue Sarcomas.
Ann Surg Oncol. 2018; 25(4):920-927 [PubMed] Related Publications
BACKGROUND: The optimal treatment of high-risk soft tissue sarcomas (STS) of the extremities remains controversial. We report follow-up from a phase II study of dose-intense chemotherapy with preoperative hypofractionated radiation in this population supplemented with subsequent data from an extensive institutional experience using this regimen.
METHODS: Patients with localized, intermediate- or high-grade STS of the extremity or body wall measuring > 5 cm were treated with epirubicin 30 mg/m
RESULTS: The 5-year rates for overall survival, distant disease-free survival, and freedom from local regional failure were 70.4% (95% CI 59.2-83.7%), 55.9% (95% CI 44.5-70.2%), and 87.2% (95% CI 77.9-96.5%) respectively. Thirty-eight percent of tumors (29/76) demonstrated ≥ 90% pathologic response. Wound complications occurred in 32% (24/76) of patients.
DISCUSSION: Treatment with preoperative radiation and pre- and post-operative epirubicin and ifosfamide was associated with favorable clinical outcomes. Survival and recurrence rates were comparable to those reported with other preoperative chemotherapy regimens in high-risk extremity sarcomas. Use of trimodality therapy should be considered for appropriate high-risk STS patients.
METHODS: Patients with localized, intermediate- or high-grade STS of the extremity or body wall measuring > 5 cm were treated with epirubicin 30 mg/m
RESULTS: The 5-year rates for overall survival, distant disease-free survival, and freedom from local regional failure were 70.4% (95% CI 59.2-83.7%), 55.9% (95% CI 44.5-70.2%), and 87.2% (95% CI 77.9-96.5%) respectively. Thirty-eight percent of tumors (29/76) demonstrated ≥ 90% pathologic response. Wound complications occurred in 32% (24/76) of patients.
DISCUSSION: Treatment with preoperative radiation and pre- and post-operative epirubicin and ifosfamide was associated with favorable clinical outcomes. Survival and recurrence rates were comparable to those reported with other preoperative chemotherapy regimens in high-risk extremity sarcomas. Use of trimodality therapy should be considered for appropriate high-risk STS patients.
Sauter CS, Matasar MJ, Schoder H, et al.
A phase 1 study of ibrutinib in combination with R-ICE in patients with relapsed or primary refractory DLBCL.
Blood. 2018; 131(16):1805-1808 [PubMed] Free Access to Full Article Related Publications
A phase 1 study of ibrutinib in combination with R-ICE in patients with relapsed or primary refractory DLBCL.
Blood. 2018; 131(16):1805-1808 [PubMed] Free Access to Full Article Related Publications
In the postrituximab era, approximately half of the patients with relapsed or refractory (rel/ref) diffuse large B-cell lymphoma (DLBCL) fail to achieve a chemosensitive response to standard salvage therapy, and are thus ineligible to proceed to autologous stem cell transplantation with curative intent. The Bruton tyrosine kinase inhibitor ibrutinib demonstrates single-agent activity in rel/ref DLBCL, particularly of non-germinal center (non-GC) cell of origin. We conducted a single-center phase 1 study evaluating dose-escalated ibrutinib, in a 3-by-3 design, in combination with rituximab, ifosfamide, carboplatin, and etoposide (R-ICE) in physiologically transplant-eligible rel/ref DLBCL patients. Twenty-one patients have been treated and are evaluable for toxicity with no dose-limiting toxicities observed through expansion with ibrutinib at 840 mg daily at dose level 3. Of the 20 patients evaluable for response, per modern International Conference on Malignant Lymphoma criteria, 11 patients achieved complete remission (CR) and 7 patients achieved partial remission for an overall response rate of 90%. All evaluable patients with non-GC DLBCL achieved a metabolic CR. Ibrutinib in combination with R-ICE demonstrates tolerability and efficacy in rel/ref DLBCL, particularly of non-GC phenotype. This treatment program warrants further investigation in later-phase studies. This trial was registered at www.clinicaltrials.gov as #NCT02219737.
Duflot T, Marie-Cardine A, Verstuyft C, et al.
Possible role of CYP2B6 genetic polymorphisms in ifosfamide-induced encephalopathy: report of three cases.
Fundam Clin Pharmacol. 2018; 32(3):337-342 [PubMed] Related Publications
Possible role of CYP2B6 genetic polymorphisms in ifosfamide-induced encephalopathy: report of three cases.
Fundam Clin Pharmacol. 2018; 32(3):337-342 [PubMed] Related Publications
Ifosfamide (IFA) is a potent alkylating antitumoral agent, but its use is limited by neurological side effects. IFA is a racemic mixture of two enantiomeric forms, R-IFA and S-IFA with a stereoselective metabolism by CYP3A4 and CYP2B6, leading either to bioactive or to toxic pathways. In three consecutive cases of pediatric patients who exhibited IFA-induced encephalopathy (IIE), genotyping of clinically relevant single-nucleotide polymorphisms associated with decreased CYP3A4 and CYP2B6 activities was performed. Genetic investigations revealed the presence of CYP2B6 rs4803419 (C>T) in one patient while the two others carried the CYP2B6*6 allelic variant. All patients carried CYP3A4 wild-type genotype (CYP3A4*1/*1). Because CYP2B6-deficient alleles may be responsible for an increased conversion of S-IFA into neurotoxic metabolites, screening for CYP2B6 polymorphisms may help to avoid IIE and improve clinical outcomes.
Brennan B, Zanetti I, Orbach D, et al.
Alveolar soft part sarcoma in children and adolescents: The European Paediatric Soft Tissue Sarcoma study group prospective trial (EpSSG NRSTS 2005).
Pediatr Blood Cancer. 2018; 65(4) [PubMed] Related Publications
Alveolar soft part sarcoma in children and adolescents: The European Paediatric Soft Tissue Sarcoma study group prospective trial (EpSSG NRSTS 2005).
Pediatr Blood Cancer. 2018; 65(4) [PubMed] Related Publications
BACKGROUND: As alveolar soft part sarcomas (ASPS) are rare with no prospective series within pediatric sarcoma trials, the European Paediatric Soft Tissue Sarcoma Study Group (EpSSG) examined the clinical data and outcomes of ASPS enrolled in a multinational study of nonrhabdomyosarcoma soft tissue sarcomas (NRSTS).
PATIENTS AND METHODS: Twenty-two patients with ASPS were enrolled into the EpSSG NRSTS 2005 study. After surgical resection, subsequent treatment depended on the stratification of patients for completeness of resection and Intergroup Rhabdomyosarcoma Study (IRS) stage, size, and French Federation of Cancer Centres Sarcoma Group (FNCLCC) grade. Chemotherapy using ifosfamide and doxorubicin was performed in IRS group III. Radiotherapy was performed in IRS groups II and III, and FNCLCC grades 2 and 3 tumors.
RESULTS: The median age at diagnosis was 11.5 years (range 2.7-17.5 years). The majority in the series had localized disease (20), with small IRS I tumors (12), and in total 19 had surgical resection upfront. Of the four patients who received conventional chemotherapy, there were no responses. Three of 20 patients with localized tumors and all metastatic patients developed metastases. The median follow up of patients with localized disease is 61.7 months (range 25.7-135.5 months) from diagnosis. The 5-year event-free survival is 94.7% (95% confidence interval: 68.1-99.2), and therefore the overall survival (OS) is 100%.
CONCLUSION: This report demonstrates the ability to run prospective pediatric studies in NRSTS in multiple European countries, despite the small numbers of ASPS patients. We can conclude that for the majority with small resected tumors, there were few events and no deaths.
PATIENTS AND METHODS: Twenty-two patients with ASPS were enrolled into the EpSSG NRSTS 2005 study. After surgical resection, subsequent treatment depended on the stratification of patients for completeness of resection and Intergroup Rhabdomyosarcoma Study (IRS) stage, size, and French Federation of Cancer Centres Sarcoma Group (FNCLCC) grade. Chemotherapy using ifosfamide and doxorubicin was performed in IRS group III. Radiotherapy was performed in IRS groups II and III, and FNCLCC grades 2 and 3 tumors.
RESULTS: The median age at diagnosis was 11.5 years (range 2.7-17.5 years). The majority in the series had localized disease (20), with small IRS I tumors (12), and in total 19 had surgical resection upfront. Of the four patients who received conventional chemotherapy, there were no responses. Three of 20 patients with localized tumors and all metastatic patients developed metastases. The median follow up of patients with localized disease is 61.7 months (range 25.7-135.5 months) from diagnosis. The 5-year event-free survival is 94.7% (95% confidence interval: 68.1-99.2), and therefore the overall survival (OS) is 100%.
CONCLUSION: This report demonstrates the ability to run prospective pediatric studies in NRSTS in multiple European countries, despite the small numbers of ASPS patients. We can conclude that for the majority with small resected tumors, there were few events and no deaths.
Tanaka I, Kawada K, Morise M, et al.
A phase II trial of Ifosfamide combination with recommended supportive therapy for recurrent SCLC in second-line and heavily treated setting.
Cancer Chemother Pharmacol. 2018; 81(2):339-345 [PubMed] Related Publications
A phase II trial of Ifosfamide combination with recommended supportive therapy for recurrent SCLC in second-line and heavily treated setting.
Cancer Chemother Pharmacol. 2018; 81(2):339-345 [PubMed] Related Publications
PURPOSE: The response rate of ifosfamide (IFM) monotherapy for small-cell lung cancer (SCLC) is reported as 42.4% in Japanese package insert. However, these efficacy data are based on clinical studies conducted in 1970s. This phase II study evaluated the efficacy and safety of IFM combination with recommended current supportive therapy for recurrent SCLC in second-line and heavily treated setting.
METHODS: Recurrent SCLC patients pretreated with one to three prior regimens received IFM monotherapy (1.5 g/m
RESULTS: Twelve patients were enrolled in the study from June 2009 to January 2013. The study was early terminated at interim analysis due to futility stop. Patient characteristics were as follows: median age was 65 years, 11 were males (91.7%) and eight (66.7%) and four (33.3%) were Performance Status 0 and 1, respectively. Four patients (33.3%) enrolled in second-line setting were all refractory relapse SCLC and 8 (66.7%) were heavily treated patients. No patient showed objective response. Stable disease was observed in 3 patients. Median progression-free survival and overall survival were 0.9 months (95% CI, 0.3-1.5) and 4.8 months (95% CI, 1.6-9.9), respectively. Although one grade 4 amylase increase possibly related to IFM was observed, toxicity profile was totally favorable.
CONCLUSIONS: IFM monotherapy should not be used for refractory relapse or heavily treated SCLC, and no further investigation is required in these populations.
METHODS: Recurrent SCLC patients pretreated with one to three prior regimens received IFM monotherapy (1.5 g/m
RESULTS: Twelve patients were enrolled in the study from June 2009 to January 2013. The study was early terminated at interim analysis due to futility stop. Patient characteristics were as follows: median age was 65 years, 11 were males (91.7%) and eight (66.7%) and four (33.3%) were Performance Status 0 and 1, respectively. Four patients (33.3%) enrolled in second-line setting were all refractory relapse SCLC and 8 (66.7%) were heavily treated patients. No patient showed objective response. Stable disease was observed in 3 patients. Median progression-free survival and overall survival were 0.9 months (95% CI, 0.3-1.5) and 4.8 months (95% CI, 1.6-9.9), respectively. Although one grade 4 amylase increase possibly related to IFM was observed, toxicity profile was totally favorable.
CONCLUSIONS: IFM monotherapy should not be used for refractory relapse or heavily treated SCLC, and no further investigation is required in these populations.
Ferrari S, Bielack SS, Smeland S, et al.
EURO-B.O.S.S.: A European study on chemotherapy in bone-sarcoma patients aged over 40: Outcome in primary high-grade osteosarcoma.
Tumori. 2018 Jan-Feb; 104(1):30-36 [PubMed] Related Publications
EURO-B.O.S.S.: A European study on chemotherapy in bone-sarcoma patients aged over 40: Outcome in primary high-grade osteosarcoma.
Tumori. 2018 Jan-Feb; 104(1):30-36 [PubMed] Related Publications
INTRODUCTION: The EUROpean Bone Over 40 Sarcoma Study (EURO-B.O.S.S.) was the first prospective international study for patients 41-65 years old with high-grade bone sarcoma treated with an intensive chemotherapy regimen derived from protocols for younger patients with high-grade skeletal osteosarcoma.
METHODS: Chemotherapy based on doxorubicin, cisplatin, ifosfamide, and methotrexate was suggested, but patients treated with other regimens at the investigators' choice were also eligible for the study.
RESULTS: The present report focuses on the subgroup of 218 patients with primary high-grade osteosarcoma. With a median follow-up of 47 months, the 5-year probability of overall survival (OS) was 66% in patients with localized disease and 22% in case of synchronous metastases. The 5-year OS in patients with localized disease was 29% in pelvic tumors, and 70% and 73% for extremity or craniofacial locations, respectively. In primary chemotherapy, tumor necrosis ≥90% was reported in 21% of the patients. There were no toxic deaths; however, hematological toxicity was considerable with 32% of patients experiencing 1 or more episodes of neutropenic fever. The incidence of nephrotoxicity and neurotoxicity (mainly peripheral) was 28% and 24%, respectively. After methotrexate, 23% of patients experienced delayed excretion, in 4 cases with nephrotoxicity.
CONCLUSIONS: In patients over 40 years of age with primary high-grade osteosarcoma, an aggressive approach with chemotherapy and surgery can offer the probability of survival similar to that achieved in younger patients. Chemotherapy-related toxicity is significant and generally higher than that reported in younger cohorts of osteosarcoma patients treated with more intensive regimens.
METHODS: Chemotherapy based on doxorubicin, cisplatin, ifosfamide, and methotrexate was suggested, but patients treated with other regimens at the investigators' choice were also eligible for the study.
RESULTS: The present report focuses on the subgroup of 218 patients with primary high-grade osteosarcoma. With a median follow-up of 47 months, the 5-year probability of overall survival (OS) was 66% in patients with localized disease and 22% in case of synchronous metastases. The 5-year OS in patients with localized disease was 29% in pelvic tumors, and 70% and 73% for extremity or craniofacial locations, respectively. In primary chemotherapy, tumor necrosis ≥90% was reported in 21% of the patients. There were no toxic deaths; however, hematological toxicity was considerable with 32% of patients experiencing 1 or more episodes of neutropenic fever. The incidence of nephrotoxicity and neurotoxicity (mainly peripheral) was 28% and 24%, respectively. After methotrexate, 23% of patients experienced delayed excretion, in 4 cases with nephrotoxicity.
CONCLUSIONS: In patients over 40 years of age with primary high-grade osteosarcoma, an aggressive approach with chemotherapy and surgery can offer the probability of survival similar to that achieved in younger patients. Chemotherapy-related toxicity is significant and generally higher than that reported in younger cohorts of osteosarcoma patients treated with more intensive regimens.
Gaspar N, Occean BV, Pacquement H, et al.
Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study.
Eur J Cancer. 2018; 88:57-66 [PubMed] Related Publications
Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study.
Eur J Cancer. 2018; 88:57-66 [PubMed] Related Publications
BACKGROUND: In most countries, reference chemotherapy for osteosarcoma is MAP regimen (M = high-dose methotrexate, AP = doxorubicin-cisplatinum). In France, the standard preoperative chemotherapy for children/adolescents combines M and etoposide-ifosfamide (EI), based on the OS94-trial. We report the safety and efficacy results of patients ≤25 years treated with preoperative M-EI regimen enroled in the French OS2006-study, between 2007 and 2014.
METHODS: Treatment comprised preoperative chemotherapy with the 7 M-courses and 2 EI-courses, then surgery and postoperative chemotherapy assigned by risk's groups: standard-risk (good histological response without metastases) received 12 M-courses, 3 EI-courses; high-risk (poor histologic response, initial metastases or unresectable primary) received 5 M-courses alternated with 5 AP-courses. 253 patients were randomised to receive (n = 128) or not (n = 125) zoledronate.
RESULTS: 409/522 patients enroled in the OS2006 study who received preoperative M-EI were analysed. Median age was 14.3 years (4.7-24.5), with 55 patients aged 18-25 years. Primary tumour location was limb in 383 patients (94%) and 85 (21%) presented metastases. Median chemotherapy duration was 37.4 weeks. 381 (96%) patients underwent surgery, 258 patients (65%) had a good histologic response. 187/324 patients (58%) with localised disease did not receive doxorubicin nor cisplatinum. Toxicity was evaluated in the randomised study: most patients experienced ≥1 severe toxicity (grade IV haematological or grade III/IV extra-haematological). Median follow-up was 4.8 years, and 168 patients had events. Five-year event-free survival was 56% (95% CI, 51-62%) and overall survival 71% (66-76%).
CONCLUSION: M-EI regimen/strategy was feasible for patient aged ≤25 years with survival rates are comparable to those obtained with MAP regimen.
METHODS: Treatment comprised preoperative chemotherapy with the 7 M-courses and 2 EI-courses, then surgery and postoperative chemotherapy assigned by risk's groups: standard-risk (good histological response without metastases) received 12 M-courses, 3 EI-courses; high-risk (poor histologic response, initial metastases or unresectable primary) received 5 M-courses alternated with 5 AP-courses. 253 patients were randomised to receive (n = 128) or not (n = 125) zoledronate.
RESULTS: 409/522 patients enroled in the OS2006 study who received preoperative M-EI were analysed. Median age was 14.3 years (4.7-24.5), with 55 patients aged 18-25 years. Primary tumour location was limb in 383 patients (94%) and 85 (21%) presented metastases. Median chemotherapy duration was 37.4 weeks. 381 (96%) patients underwent surgery, 258 patients (65%) had a good histologic response. 187/324 patients (58%) with localised disease did not receive doxorubicin nor cisplatinum. Toxicity was evaluated in the randomised study: most patients experienced ≥1 severe toxicity (grade IV haematological or grade III/IV extra-haematological). Median follow-up was 4.8 years, and 168 patients had events. Five-year event-free survival was 56% (95% CI, 51-62%) and overall survival 71% (66-76%).
CONCLUSION: M-EI regimen/strategy was feasible for patient aged ≤25 years with survival rates are comparable to those obtained with MAP regimen.
Meazza C, Cefalo G, Massimino M, et al.
Primary metastatic osteosarcoma: results of a prospective study in children given chemotherapy and interleukin-2.
Med Oncol. 2017; 34(12):191 [PubMed] Related Publications
Primary metastatic osteosarcoma: results of a prospective study in children given chemotherapy and interleukin-2.
Med Oncol. 2017; 34(12):191 [PubMed] Related Publications
To improve the poor prognosis for children with metastatic osteosarcoma (OS), interleukin-2 (IL-2) was added to the standard treatment due to its capacity to activate lymphocytes and differentiate lymphocyte subsets into lymphokine-activated killer (LAK) cells that are capable of recognizing and killing various tumor cells. This study concerns a cohort of unselected patients aged < 18 years with metastatic OS, who were treated with IL-2, high-dose methotrexate, doxorubicin, cisplatin, ifosfamide, LAK reinfusion, and surgery, between 1995 and 2010. Thirty-five patients aged 4-17 years were involved. Thirty-two of the 35 patients underwent surgery on their primary tumor, and 25 had surgery on lung metastases too. Twenty-seven patients received IL-2 plus LAK reinfusion. The median follow-up was 130 months (77-228), and the 3-year event-free and overall survival rates were 34.3 and 45.0%, respectively. Eleven patients remained alive, all of whom achieved a complete surgical removal of the primary tumor and lung metastases (1 patient did not receive lung resections due to complete lung metastases remission). Patients who had a complete surgical remission of the primary and metastatic sites and who responded well to chemotherapy had a better event-free survival. These results confirm the importance of complete surgical remission and point to a noteworthy (though still be ameliorate) survival rate in our series of patients, underling a potential role for immunotherapy with IL-2 and LAK/NK cell activation.
Federico SM, McCarville MB, Shulkin BL, et al.
A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma.
Clin Cancer Res. 2017; 23(21):6441-6449 [PubMed] Related Publications
A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma.
Clin Cancer Res. 2017; 23(21):6441-6449 [PubMed] Related Publications
Seno N, Fukushima T, Gomi D, et al.
Successful treatment with doxorubicin and ifosfamide for mediastinal malignant peripheral nerve sheath tumor with loss of H3K27me3 expression.
Thorac Cancer. 2017; 8(6):720-723 [PubMed] Free Access to Full Article Related Publications
Successful treatment with doxorubicin and ifosfamide for mediastinal malignant peripheral nerve sheath tumor with loss of H3K27me3 expression.
Thorac Cancer. 2017; 8(6):720-723 [PubMed] Free Access to Full Article Related Publications
Malignant peripheral nerve sheath tumor (MPNST) in the thorax is an extremely rare disease, and half of all cases of MPNST are associated with neurofibromatosis type I. Sporadic intrathoracic MPNST is difficult to diagnose and treat. Because of the rarity of intrathoracic MPNST, the optimal method of diagnosis and the efficacy of chemotherapy are unknown. Herein, we present a case of inoperable mediastinal MPNST, in which the diagnosis was immunohistochemically made by the loss of H3K27me3 expression in a transbronchial needle biopsy specimen. The patient showed a good response to doxorubicin plus ifosfamide chemotherapy. The present case highlights that MPNST should be included in the differential diagnosis of non-posterior mediastinum thoracic lesions, and that appropriate diagnosis and treatment for intrathoracic MPNST should be considered in patients with a thoracic mass.
Hu B, Younes A, Westin JR, et al.
Phase-I and randomized phase-II trial of panobinostat in combination with ICE (ifosfamide, carboplatin, etoposide) in relapsed or refractory classical Hodgkin lymphoma.
Leuk Lymphoma. 2018; 59(4):863-870 [PubMed] Related Publications
Phase-I and randomized phase-II trial of panobinostat in combination with ICE (ifosfamide, carboplatin, etoposide) in relapsed or refractory classical Hodgkin lymphoma.
Leuk Lymphoma. 2018; 59(4):863-870 [PubMed] Related Publications
This phase-I/phase-II study evaluated panobinostat in combination with ifosfamide, carboplatin, etoposide (P-ICE) in relapsed/refractory classical Hodgkin lymphoma. During phase I, panobinostat was given daily on Monday/Wednesday/Friday starting one week prior to Cycle 1 (C1) of ICE and during two weeks of C1-2 of ICE (Schedule A). No DLT was observed at 30 mg. However, frequent (84%) grade-4 thrombocytopenia during second week prompted us to omit the second week of panobinostat 30 mg (Schedule B) for phase II, where this regimen was compared to ICE. In the randomized phase-II study, CR was seen in 9/11 (82%) and 8/12 (67%) for P-ICE and ICE, respectively (p = .64). Grade-4 neutropenia (55% vs. 8%) and thrombocytopenia (100% vs. 33%) were more common in P-ICE. In summary, combination therapy using panobinostat produced high CR rate at the cost of greater bone marrow toxicity. Investigation of panobinostat with less myelosuppressive agents is of interest.
Wagner MJ, Gopalakrishnan V, Ravi V, et al.
Vincristine, Ifosfamide, and Doxorubicin for Initial Treatment of Ewing Sarcoma in Adults.
Oncologist. 2017; 22(10):1271-1277 [PubMed] Free Access to Full Article Related Publications
Vincristine, Ifosfamide, and Doxorubicin for Initial Treatment of Ewing Sarcoma in Adults.
Oncologist. 2017; 22(10):1271-1277 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: There are no clinical trials specifically addressing chemotherapy for adults with Ewing sarcoma (ES). Five-year event-free survival (EFS) of adults on pediatric studies of ES (44%-47%) is worse than that of children treated with the same therapy (69%). The object of this study was to review the results of therapy with vincristine, ifosfamide, and doxorubicin (VID) in the multidisciplinary treatment of adults with ES at our institution.
MATERIALS AND METHODS: Charts for adults treated for ES from 1995 to 2011 were retrospectively reviewed. Clinician-reported radiographic tumor response, type of local therapy, pathologic response, and survival data were collected.
RESULTS: Seventy-one patients were identified who received VID as initial therapy. The median age was 25 (range: 16-64). Forty-two patients (59%) presented with a localized disease and 29 patients (41%) presented with a distant metastasis. Of all patients treated with VID, 83.6% showed a radiological response. Patients who presented with a localized disease had a 5-year overall survival (OS) of 68% (median not reached), compared with 10.3% (median: 1.9 years) in those who presented with distant metastases. Five-year EFS was 67%. The nine patients with a pelvic primary tumor had inferior 5-year OS (42%) to the 33 with primary tumors at other sites (75%). The 5-year OS of those who had greater than or equal to 95% necrosis after neoadjuvant VID (
CONCLUSION: In adults with primary ES, VID combined with an adjuvant strategy based on post-treatment percent necrosis has favorable outcomes compared with historical adult controls.
IMPLICATIONS FOR PRACTICE: Ewing sarcoma (ES) is a rare tumor in adults, and there are no dedicated clinical trials in the adult population. Most therapy is modeled after the published pediatric studies, although the small numbers of adult patients included on those studies did significantly worse than the children. We modeled our treatment on other adult sarcomas and reviewed the charts of 71 adult patients with ES treated with vincristine, ifosfamide, and doxorubicin (VID). In adults with primary ES, VID combined with an adjuvant strategy based on post-treatment percent necrosis has favorable outcomes compared with historical adult controls.
MATERIALS AND METHODS: Charts for adults treated for ES from 1995 to 2011 were retrospectively reviewed. Clinician-reported radiographic tumor response, type of local therapy, pathologic response, and survival data were collected.
RESULTS: Seventy-one patients were identified who received VID as initial therapy. The median age was 25 (range: 16-64). Forty-two patients (59%) presented with a localized disease and 29 patients (41%) presented with a distant metastasis. Of all patients treated with VID, 83.6% showed a radiological response. Patients who presented with a localized disease had a 5-year overall survival (OS) of 68% (median not reached), compared with 10.3% (median: 1.9 years) in those who presented with distant metastases. Five-year EFS was 67%. The nine patients with a pelvic primary tumor had inferior 5-year OS (42%) to the 33 with primary tumors at other sites (75%). The 5-year OS of those who had greater than or equal to 95% necrosis after neoadjuvant VID (
CONCLUSION: In adults with primary ES, VID combined with an adjuvant strategy based on post-treatment percent necrosis has favorable outcomes compared with historical adult controls.
IMPLICATIONS FOR PRACTICE: Ewing sarcoma (ES) is a rare tumor in adults, and there are no dedicated clinical trials in the adult population. Most therapy is modeled after the published pediatric studies, although the small numbers of adult patients included on those studies did significantly worse than the children. We modeled our treatment on other adult sarcomas and reviewed the charts of 71 adult patients with ES treated with vincristine, ifosfamide, and doxorubicin (VID). In adults with primary ES, VID combined with an adjuvant strategy based on post-treatment percent necrosis has favorable outcomes compared with historical adult controls.
Kim HJ, Ock CY, Kim TM, et al.
Comparison of Native Escherichia coli L-Asparaginase versus Pegylated Asparaginase, in Combination with Ifosfamide, Methotrexate, Etoposide, and Prednisolone, in Extranodal NK/T-Cell Lymphoma, Nasal Type.
Cancer Res Treat. 2018; 50(3):670-680 [PubMed] Free Access to Full Article Related Publications
Comparison of Native Escherichia coli L-Asparaginase versus Pegylated Asparaginase, in Combination with Ifosfamide, Methotrexate, Etoposide, and Prednisolone, in Extranodal NK/T-Cell Lymphoma, Nasal Type.
Cancer Res Treat. 2018; 50(3):670-680 [PubMed] Free Access to Full Article Related Publications
Purpose: The aim of this study was to compare asparaginase-related toxicities in two asparaginase preparations, namely native Escherichia coli L-asparaginase (L-ASP) and pegylated asparaginase (PEG-ASP) in combination with ifosfamide, methotrexate, etoposide, and prednisolone (IMEP) in natural killer (NK)/T-cell lymphoma (NTCL).
Materials and Methods: A total of 41 NTCL patients who received IMEP plus native E. coli L-ASP or PEG-ASP at Seoul National University Hospital were included in this study between January 2013 and March 2016. IMEP/ASP treatment consisted of ifosfamide, methotrexate, etoposide, plus native E. coli L-ASP (6,000 IU/m2 on days 1, 3, 5, 7, 9, and 11) or PEG-ASP (2,500 IU/m2 on day 1) every 3 weeks. ASP-related toxicities, toxicity patterns, length of hospital stay, and clinical outcomes were compared between the different treatment groups.
Results: The frequency of ASP-related toxicities was similar between the IMEP plus native E. coli L-ASP group and the PEG-ASP group apart from hypofibrinogenemia (native E. coli L-ASP vs. PEG-ASP group, 86.4% vs. 36.8%; p=0.001). Although post-treatment transaminase and albumin levels were significantly high and low, respectively, hepatotoxicity gradients before and after treatment did not differ significantly between the groups. Since PEG-ASP was given at an outpatient clinic in some patients, length of hospital stay was significantly shorter in the IMEP plus PEG-ASP group (median, 4.0 vs. 6.0 days; p=0.002). A favorable tendency of clinical outcomes was observed in NTCL patients treated with IMEP plus PEG-ASP (complete remission rate, 73.7% vs. 45.5%; p=0.067).
Conclusion: IMEP plus PEG-ASP showed similar ASP-related toxicities, shorter length of hospital stay, and a trend towards improved clinical outcomes compared with IMEP plus native E. coli L-ASP in NTCL.
Materials and Methods: A total of 41 NTCL patients who received IMEP plus native E. coli L-ASP or PEG-ASP at Seoul National University Hospital were included in this study between January 2013 and March 2016. IMEP/ASP treatment consisted of ifosfamide, methotrexate, etoposide, plus native E. coli L-ASP (6,000 IU/m2 on days 1, 3, 5, 7, 9, and 11) or PEG-ASP (2,500 IU/m2 on day 1) every 3 weeks. ASP-related toxicities, toxicity patterns, length of hospital stay, and clinical outcomes were compared between the different treatment groups.
Results: The frequency of ASP-related toxicities was similar between the IMEP plus native E. coli L-ASP group and the PEG-ASP group apart from hypofibrinogenemia (native E. coli L-ASP vs. PEG-ASP group, 86.4% vs. 36.8%; p=0.001). Although post-treatment transaminase and albumin levels were significantly high and low, respectively, hepatotoxicity gradients before and after treatment did not differ significantly between the groups. Since PEG-ASP was given at an outpatient clinic in some patients, length of hospital stay was significantly shorter in the IMEP plus PEG-ASP group (median, 4.0 vs. 6.0 days; p=0.002). A favorable tendency of clinical outcomes was observed in NTCL patients treated with IMEP plus PEG-ASP (complete remission rate, 73.7% vs. 45.5%; p=0.067).
Conclusion: IMEP plus PEG-ASP showed similar ASP-related toxicities, shorter length of hospital stay, and a trend towards improved clinical outcomes compared with IMEP plus native E. coli L-ASP in NTCL.
Karageorgopoulou S, Kostakis ID, Gazouli M, et al.
Prognostic and predictive factors in patients with metastatic or recurrent cervical cancer treated with platinum-based chemotherapy.
BMC Cancer. 2017; 17(1):451 [PubMed] Free Access to Full Article Related Publications
Prognostic and predictive factors in patients with metastatic or recurrent cervical cancer treated with platinum-based chemotherapy.
BMC Cancer. 2017; 17(1):451 [PubMed] Free Access to Full Article Related Publications
BACKGROUND: Recognizing resistance or susceptibility to the current standard cisplatin and paclitaxel treatment could improve therapeutic outcomes of metastatic or recurrent cervical cancer.
METHODS: Forty-five tissue samples from patients participating in a phase II trial of cisplatin and ifosfamide, with or without paclitaxel were collected for retrograde analysis. Immunohistochemistry and genotyping was performed to test ERCC1, III β-tubulin, COX-2, CD4, CD8 and ERCC1 (C8092A and N118 N) and MDR1 (C3435T and G2677 T) gene polymorphisms, as possible predictive and prognostic markers. Results were statistically analyzed and correlated with patient characteristics and outcomes.
RESULTS: Patients with higher levels of ERCC1 expression had shorter PFS and OS than patients with low ERCC1 expression (mPFS:5.1 vs 10.2 months, p = 0.027; mOS:10.5 vs. 21.4 months, p = 0.006). Patients with TT in the site of ERCC1 N118 N and GT in the site of MDR1 G2677 T polymorphisms had significantly longer PFS (p = 0.006 and p = 0.027 respectively). ERCC1 expression and the ERCC1 N118 N polymorphism remained independent predictors of PFS. Interestingly, high III beta tubulin expression was associated with chemotherapy resistance and fewer responses [5/20 (25%)] compared to lower III β-tubulin expression [15/23 (65.2%)] (p = 0.008). Finally, ΙΙΙ β-tubulin levels and chemotherapy regimen were independent predictors of response to treatment.
CONCLUSIONS: ERCC1 expression proved to be a significant prognostic factor for survival in our metastatic or recurrent cervical cancer population treated with cisplatin based chemotherapy. ERCC1 N118 N and MDR1 G2677 T polymorphism also proved of prognostic significance for disease progression, while overexpression of III β-tubulin was positively correlated with chemotherapy resistance.
METHODS: Forty-five tissue samples from patients participating in a phase II trial of cisplatin and ifosfamide, with or without paclitaxel were collected for retrograde analysis. Immunohistochemistry and genotyping was performed to test ERCC1, III β-tubulin, COX-2, CD4, CD8 and ERCC1 (C8092A and N118 N) and MDR1 (C3435T and G2677 T) gene polymorphisms, as possible predictive and prognostic markers. Results were statistically analyzed and correlated with patient characteristics and outcomes.
RESULTS: Patients with higher levels of ERCC1 expression had shorter PFS and OS than patients with low ERCC1 expression (mPFS:5.1 vs 10.2 months, p = 0.027; mOS:10.5 vs. 21.4 months, p = 0.006). Patients with TT in the site of ERCC1 N118 N and GT in the site of MDR1 G2677 T polymorphisms had significantly longer PFS (p = 0.006 and p = 0.027 respectively). ERCC1 expression and the ERCC1 N118 N polymorphism remained independent predictors of PFS. Interestingly, high III beta tubulin expression was associated with chemotherapy resistance and fewer responses [5/20 (25%)] compared to lower III β-tubulin expression [15/23 (65.2%)] (p = 0.008). Finally, ΙΙΙ β-tubulin levels and chemotherapy regimen were independent predictors of response to treatment.
CONCLUSIONS: ERCC1 expression proved to be a significant prognostic factor for survival in our metastatic or recurrent cervical cancer population treated with cisplatin based chemotherapy. ERCC1 N118 N and MDR1 G2677 T polymorphism also proved of prognostic significance for disease progression, while overexpression of III β-tubulin was positively correlated with chemotherapy resistance.
Aznab M, Hematti M
Evaluation of clinical process in osteosarcoma patients treated with chemotherapy including cisplatin, adriamycin, ifosfamide, and etoposide and determination of the treatment sequels in a long-term 11-year follow-up.
J Cancer Res Ther. 2017 Apr-Jun; 13(2):291-296 [PubMed] Related Publications
Evaluation of clinical process in osteosarcoma patients treated with chemotherapy including cisplatin, adriamycin, ifosfamide, and etoposide and determination of the treatment sequels in a long-term 11-year follow-up.
J Cancer Res Ther. 2017 Apr-Jun; 13(2):291-296 [PubMed] Related Publications
AIM: The aim of this study was to evaluate the effect of adding etoposide and ifosfamide chemotherapy drugs to treatment regimen of patients affected with osteosarcoma and to determine the clinical process and response to treatment during a follow-up period of 11 years.
MATERIALS AND METHODS: Forty patients with osteosarcoma participated in this study from July 2005 to 2016. Treatments were started based on the following schema and after initial examinations including biochemical profile, checking for lung metastasis by simple radiography, chest computed tomography scan, and bones scan. The initial chemotherapy which consisted of four cycles of cisplatin and adriamycin alternative with ifosfamide and etoposide was provided. Afterward, resection of the primary tumor and also the metastatic lesions was performed in patients with lung metastasis in case they had radiological evidence of response to the treatment.
RESULTS: The mean of follow-up duration in this study was 50 months. Thirty-four patients did not have metastasis and six were metastatic. Of 34 patients, 18 had relapse and 16 patients never had relapse. Five patients experienced only local relapse, nine had only systemic relapse, and two patients had simultaneous systemic and local relapses. Thirteen patients had died. The mean of overall survival in patients was 81 months. The mean of survival in patients with and without primary metastasis was 30 and 90 months, respectively.
CONCLUSION: Favorable response to the treatment was obtained with an appropriate multiple disciplinary works in the osteosarcomas of extremities, and there were no considerable side effects and sequels in the long-term follow-up of these treatments.
MATERIALS AND METHODS: Forty patients with osteosarcoma participated in this study from July 2005 to 2016. Treatments were started based on the following schema and after initial examinations including biochemical profile, checking for lung metastasis by simple radiography, chest computed tomography scan, and bones scan. The initial chemotherapy which consisted of four cycles of cisplatin and adriamycin alternative with ifosfamide and etoposide was provided. Afterward, resection of the primary tumor and also the metastatic lesions was performed in patients with lung metastasis in case they had radiological evidence of response to the treatment.
RESULTS: The mean of follow-up duration in this study was 50 months. Thirty-four patients did not have metastasis and six were metastatic. Of 34 patients, 18 had relapse and 16 patients never had relapse. Five patients experienced only local relapse, nine had only systemic relapse, and two patients had simultaneous systemic and local relapses. Thirteen patients had died. The mean of overall survival in patients was 81 months. The mean of survival in patients with and without primary metastasis was 30 and 90 months, respectively.
CONCLUSION: Favorable response to the treatment was obtained with an appropriate multiple disciplinary works in the osteosarcomas of extremities, and there were no considerable side effects and sequels in the long-term follow-up of these treatments.
Salihi R, Leunen K, Moerman P, et al.
Neoadjuvant Weekly Paclitaxel-Carboplatin Is Effective in Stage I-II Cervical Cancer.
Int J Gynecol Cancer. 2017; 27(6):1256-1260 [PubMed] Related Publications
Neoadjuvant Weekly Paclitaxel-Carboplatin Is Effective in Stage I-II Cervical Cancer.
Int J Gynecol Cancer. 2017; 27(6):1256-1260 [PubMed] Related Publications
OBJECTIVE: Neoadjuvant chemotherapy (NACT) followed by surgery in cervical cancer is widely studied with paclitaxel-ifosfamide-cisplatinum 3 weekly (TIP). Although the response rates with TIP are high, the toxicity is substantial. Therefore, this study evaluates dose-dense paclitaxel-carboplatin (TC) as an alternative.
METHODS: In this prospective phase 2 study trial, we included 36 patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB1 to IIB cervical cancer, who received 9 weeks' NACT dose-dense TC (median weekly dose paclitaxel 60 mg/m, carboplatinum area under the curve 2.7). Radiological response was evaluated by RECIST (Response Evaluation Criteria in Solid Tumors). Optimal pathologic response (OPT) was defined as complete disappearance of tumor (complete response [CR]) or residual disease with less than 3-mm stromal invasion (PR1). Suboptimal pathologic response consisted of persistent residual disease with more than 3-mm stromal invasion (PR2).
RESULTS: Nine patients had a FIGO stage IB1 (25%), 7 had stage IB2(19%), 3 had stage IIA (8%), and 17 had stage IIB disease (47%). Evaluation by magnetic resonance imaging after NACT showed 32 RECIST responses (89%) (CR in 11, PR in 21). Patients who were inoperable had insufficient reduction of the tumor to be operable (4 patients), progressive disease (1 patient), or stable disease (1 patient). Thirty patients were suitable for surgery after NACT. Pathology showed OPT in 50% (CR in 10, PR1 in 5). Thirteen patients had pathologic lymph nodes on radiological evaluation before start of chemotherapy. After chemotherapy, the lymph nodes were negative in 6 (47%) of these patients (pathologic complete remission). Postoperative chemoradiotherapy was administered in 11 patients (2 because of close resection margins, 5 because of metastatic lymph node after surgery, 2 because of close resection margins and metastatic lymph nodes after surgery, and 1 tumor >4 cm after NACT). Hematologic toxicity was acceptable with no febrile neutropenia and a low nonhematologic toxicity. The estimated 5-year overall survival was 70.8%.
CONCLUSIONS: Neoadjuvant TC dose-dense in cervical carcinoma has a high response rate, comparable with TIP, and an acceptable toxicity.
METHODS: In this prospective phase 2 study trial, we included 36 patients with International Federation of Gynecology and Obstetrics (FIGO) stage IB1 to IIB cervical cancer, who received 9 weeks' NACT dose-dense TC (median weekly dose paclitaxel 60 mg/m, carboplatinum area under the curve 2.7). Radiological response was evaluated by RECIST (Response Evaluation Criteria in Solid Tumors). Optimal pathologic response (OPT) was defined as complete disappearance of tumor (complete response [CR]) or residual disease with less than 3-mm stromal invasion (PR1). Suboptimal pathologic response consisted of persistent residual disease with more than 3-mm stromal invasion (PR2).
RESULTS: Nine patients had a FIGO stage IB1 (25%), 7 had stage IB2(19%), 3 had stage IIA (8%), and 17 had stage IIB disease (47%). Evaluation by magnetic resonance imaging after NACT showed 32 RECIST responses (89%) (CR in 11, PR in 21). Patients who were inoperable had insufficient reduction of the tumor to be operable (4 patients), progressive disease (1 patient), or stable disease (1 patient). Thirty patients were suitable for surgery after NACT. Pathology showed OPT in 50% (CR in 10, PR1 in 5). Thirteen patients had pathologic lymph nodes on radiological evaluation before start of chemotherapy. After chemotherapy, the lymph nodes were negative in 6 (47%) of these patients (pathologic complete remission). Postoperative chemoradiotherapy was administered in 11 patients (2 because of close resection margins, 5 because of metastatic lymph node after surgery, 2 because of close resection margins and metastatic lymph nodes after surgery, and 1 tumor >4 cm after NACT). Hematologic toxicity was acceptable with no febrile neutropenia and a low nonhematologic toxicity. The estimated 5-year overall survival was 70.8%.
CONCLUSIONS: Neoadjuvant TC dose-dense in cervical carcinoma has a high response rate, comparable with TIP, and an acceptable toxicity.
Gronchi A, Ferrari S, Quagliuolo V, et al.
Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial.
Lancet Oncol. 2017; 18(6):812-822 [PubMed] Related Publications
Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): an international, open-label, randomised, controlled, phase 3, multicentre trial.
Lancet Oncol. 2017; 18(6):812-822 [PubMed] Related Publications
BACKGROUND: Previous trials from our group suggested an overall survival benefit with five cycles of adjuvant full-dose epirubicin plus ifosfamide in localised high-risk soft-tissue sarcoma of the extremities or trunk wall, and no difference in overall survival benefit between three cycles versus five cycles of the same neoadjuvant regimen. We aimed to show the superiority of the neoadjuvant administration of histotype-tailored regimen to standard chemotherapy.
METHODS: For this international, open-label, randomised, controlled, phase 3, multicentre trial, patients were enrolled from 32 hospitals in Italy, Spain, France, and Poland. Eligible patients were aged 18 years or older with localised, high-risk (high malignancy grade, 5 cm or longer in diameter, and deeply located according to the investing fascia), soft-tissue sarcoma of the extremities or trunk wall and belonging to one of five histological subtypes: high-grade myxoid liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumour, and undifferentiated pleomorphic sarcoma. Patients were randomly assigned (1:1) to receive three cycles of full-dose standard chemotherapy (epirubicin 60 mg/m
FINDINGS: Between May 19, 2011, and May 13, 2016, 287 patients were randomly assigned to a group (145 to standard chemotherapy and 142 to histotype-tailored chemotherapy), all of whom, except one patient assigned to standard chemotherapy, were included in the efficacy analysis (97 [34%] with undifferentiated pleomorphic sarcoma; 64 [22%] with high-grade myxoid liposarcoma; 70 [24%] with synovial sarcoma; 27 [9%] with malignant peripheral nerve sheath tumour; and 28 [10%] with leiomyosarcoma). At the third futility analysis, with a median follow-up of 12·3 months (IQR 2·75-28·20), the projected disease-free survival at 46 months was 62% (95% CI 48-77) in the standard chemotherapy group and 38% (22-55) in the histotype-tailored chemotherapy group (stratified log-rank p=0·004; hazard ratio 2·00, 95% CI 1·22-3·26; p=0·006). The most common grade 3 or higher adverse events in the standard chemotherapy group (n=125) were neutropenia (107 [86%]), anaemia (24 [19%]), and thrombocytopenia (21 [17%]); the most common grade 3 or higher adverse event in the histotype-tailored chemotherapy group (n=114) was neutropenia (30 [26%]). No treatment-related deaths were reported in both groups. In agreement with the Independent Data Monitoring Committee, the study was closed to patient entry after the third futility analysis.
INTERPRETATION: In a population of patients with high-risk soft-tissue sarcoma, we did not show any benefit of a neoadjuvant histotype-tailored chemotherapy regimen over the standard chemotherapy regimen. The benefit seen with the standard chemotherapy regimen suggests that this benefit might be the added value of neoadjuvant chemotherapy itself in patients with high-risk soft-tissue sarcoma.
FUNDING: European Union grant (Eurosarc FP7 278472).
METHODS: For this international, open-label, randomised, controlled, phase 3, multicentre trial, patients were enrolled from 32 hospitals in Italy, Spain, France, and Poland. Eligible patients were aged 18 years or older with localised, high-risk (high malignancy grade, 5 cm or longer in diameter, and deeply located according to the investing fascia), soft-tissue sarcoma of the extremities or trunk wall and belonging to one of five histological subtypes: high-grade myxoid liposarcoma, leiomyosarcoma, synovial sarcoma, malignant peripheral nerve sheath tumour, and undifferentiated pleomorphic sarcoma. Patients were randomly assigned (1:1) to receive three cycles of full-dose standard chemotherapy (epirubicin 60 mg/m
FINDINGS: Between May 19, 2011, and May 13, 2016, 287 patients were randomly assigned to a group (145 to standard chemotherapy and 142 to histotype-tailored chemotherapy), all of whom, except one patient assigned to standard chemotherapy, were included in the efficacy analysis (97 [34%] with undifferentiated pleomorphic sarcoma; 64 [22%] with high-grade myxoid liposarcoma; 70 [24%] with synovial sarcoma; 27 [9%] with malignant peripheral nerve sheath tumour; and 28 [10%] with leiomyosarcoma). At the third futility analysis, with a median follow-up of 12·3 months (IQR 2·75-28·20), the projected disease-free survival at 46 months was 62% (95% CI 48-77) in the standard chemotherapy group and 38% (22-55) in the histotype-tailored chemotherapy group (stratified log-rank p=0·004; hazard ratio 2·00, 95% CI 1·22-3·26; p=0·006). The most common grade 3 or higher adverse events in the standard chemotherapy group (n=125) were neutropenia (107 [86%]), anaemia (24 [19%]), and thrombocytopenia (21 [17%]); the most common grade 3 or higher adverse event in the histotype-tailored chemotherapy group (n=114) was neutropenia (30 [26%]). No treatment-related deaths were reported in both groups. In agreement with the Independent Data Monitoring Committee, the study was closed to patient entry after the third futility analysis.
INTERPRETATION: In a population of patients with high-risk soft-tissue sarcoma, we did not show any benefit of a neoadjuvant histotype-tailored chemotherapy regimen over the standard chemotherapy regimen. The benefit seen with the standard chemotherapy regimen suggests that this benefit might be the added value of neoadjuvant chemotherapy itself in patients with high-risk soft-tissue sarcoma.
FUNDING: European Union grant (Eurosarc FP7 278472).


 Epirubicin
Epirubicin