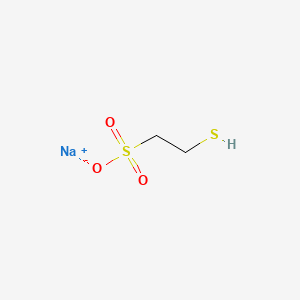
Mesna
"A sulfhydryl compound used to prevent urothelial toxicity by inactivating metabolites from ANTINEOPLASTIC AGENTS, such as IFOSFAMIDE or CYCLOPHOSPHAMIDE." (MeSH 2013)
Found this page useful?
 Web Resources: Mesna
Web Resources: Mesna Latest Research Publications
Latest Research PublicationsWeb Resources: Mesna (6 links)
Cancer Research UK
Irish Cancer Society
Macmillan Cancer Support
MedlinePlus
NHS Evidence
PubChem
Latest Research Publications
This list of publications is regularly updated (Source: PubMed).
Lee J, Jung HA, Kim Y, et al.
Efficacy of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in patients with advanced pulmonary pleomorphic carcinoma.
Lung Cancer. 2018; 122:160-164 [PubMed] Related Publications
Efficacy of mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) in patients with advanced pulmonary pleomorphic carcinoma.
Lung Cancer. 2018; 122:160-164 [PubMed] Related Publications
OBJECTIVES: Pulmonary pleomorphic carcinoma (PC) is a rare type of lung tumor with a dismal prognosis. There is no consensus on a chemotherapy regimen for PC, and conventional platinum-based chemotherapy has been associated with disappointing response rates and PFS. In searches for a new regimen, the sarcomatoid (spindle or giant cell) component has been assumed to be susceptible to chemotherapy used for soft tissue sarcoma.
MATERIALS AND METHODS: The medical records of 17 patients who received mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) for advanced PC between January 2010 and February 2017 were retrospectively analyzed for clinicopathological features and outcomes.
RESULTS AND CONCLUSION: The median age was 59 years. Sixteen patients were male, and only one patient had never smoked. Six patients achieved partial response to MAID, leading to an objective response rate of 35%. The median PFS was 2.8 months, and the median OS was 8.7 months. Hematologic toxicity-related adverse events were the most frequent, which comprised grade 3-4 anemia in 35% of patients, neutropenia in 47%, thrombocytopenia in 24%, and febrile neutropenia in 29%. No febrile neutropenia was reported in patients who received 5-day granulocyte-colony stimulating factor (G-CSF) prophylaxis. Most adverse events resolved without complications, except for one death due to sepsis. MAID is an effective, and possibly important, regimen for PC. MAID could be more safely used in clinical practice with appropriate dose modifications and G-CSF primary prophylaxis according to patients' status.
MATERIALS AND METHODS: The medical records of 17 patients who received mesna, doxorubicin, ifosfamide, and dacarbazine (MAID) for advanced PC between January 2010 and February 2017 were retrospectively analyzed for clinicopathological features and outcomes.
RESULTS AND CONCLUSION: The median age was 59 years. Sixteen patients were male, and only one patient had never smoked. Six patients achieved partial response to MAID, leading to an objective response rate of 35%. The median PFS was 2.8 months, and the median OS was 8.7 months. Hematologic toxicity-related adverse events were the most frequent, which comprised grade 3-4 anemia in 35% of patients, neutropenia in 47%, thrombocytopenia in 24%, and febrile neutropenia in 29%. No febrile neutropenia was reported in patients who received 5-day granulocyte-colony stimulating factor (G-CSF) prophylaxis. Most adverse events resolved without complications, except for one death due to sepsis. MAID is an effective, and possibly important, regimen for PC. MAID could be more safely used in clinical practice with appropriate dose modifications and G-CSF primary prophylaxis according to patients' status.
Dandamudi RK, Aslam S, Walji N, et al.
Chemotherapy for Uterine Carcinosarcoma with Carboplatin, Ifosfamide and Mesna.
Anticancer Res. 2015; 35(9):4841-7 [PubMed] Related Publications
Chemotherapy for Uterine Carcinosarcoma with Carboplatin, Ifosfamide and Mesna.
Anticancer Res. 2015; 35(9):4841-7 [PubMed] Related Publications
BACKGROUND/AIM: Uterine carcinosarcomas (UCSs) are highly aggressive, rare, biphasic tumors composed of epithelial and mesenchymal elements. Surgery remains the mainstay of treatment in early-stage disease. Adjuvant pelvic radiotherapy improves locoregional control without proven overall survival (OS) benefit. Although adjuvant ifosfamide-based combination chemotherapy with cisplatin or paclitaxel has shown superiority to radiotherapy or single-agent chemotherapy in randomized controlled trials, there is no consensus on a standard regimen due to toxicities. The aim of this retrospective study was to assess the efficacy and toxicity of a novel combination chemotherapy using carboplatin, ifosfamide and mesna (CIM) and compare with other regimens for patients with UCSs in both the adjuvant and palliative setting.
PATIENTS AND METHODS: Between 1997 and 2010, 60 patients with UCS, 70% of whom with international federation of gynecology and obstetrics (FIGO) stage III/IV disease, were treated with adjuvant or palliative chemotherapy. Two groups were identified: Group1 (n=22) included patients receiving CIM chemotherapy; and group 2 (n=38) receiving other regimens (carboplatin/paclitaxel/cisplatin/doxorubicin/epirubicin).
RESULTS: After a median follow-up of 60 months, disease in seven patients in group 1 (CIM) and 20 patients in group 2 had progressed/relapsed. Out of these, six patients in group 1 and 13 patients in group 2 had died. The progression-free survival (PFS) and OS for patients treated with adjuvant or palliative CIM was 35 months [95% confidence interval (CI) =0.26-0.43] and 47 months (95% CI=0.38-0.56; log-rank, p=0.001) respectively, whereas for group 2 patients treated with other regimens, PFS was 27.48 months (95% CI=0.20-0.33) and OS was 30 months (95% CI=0.21-0.38; log-rank, p=0.001). While none of the patients in group 1 experienced neurotoxicity or other grade 3 or 4 toxicities, 3/38 patients in group 2 experienced grade 3 neutropenia, 4/38 had peripheral sensory neuropathy, 6/38 patients had treatment deferred due to toxicities or allergic reaction to paclitaxel.
CONCLUSION: In the phase III randomized controlled trial combination of ifosfamide and taxanes has shown PFS and OS benefit when compared to single-agent ifosfamide at the expense of significant toxicities. Results from our study show that the combination of CIM is an effective and safe alternative regimen for patients with advanced UCSs. In addition to improved OS and PFS, the main advantage of this regimen over taxane-based regimens includes minimal neuropathy, less use of steroids, and low risk of allergic reaction. CIM should be considered in future prospective studies looking at the treatment of UCS.
PATIENTS AND METHODS: Between 1997 and 2010, 60 patients with UCS, 70% of whom with international federation of gynecology and obstetrics (FIGO) stage III/IV disease, were treated with adjuvant or palliative chemotherapy. Two groups were identified: Group1 (n=22) included patients receiving CIM chemotherapy; and group 2 (n=38) receiving other regimens (carboplatin/paclitaxel/cisplatin/doxorubicin/epirubicin).
RESULTS: After a median follow-up of 60 months, disease in seven patients in group 1 (CIM) and 20 patients in group 2 had progressed/relapsed. Out of these, six patients in group 1 and 13 patients in group 2 had died. The progression-free survival (PFS) and OS for patients treated with adjuvant or palliative CIM was 35 months [95% confidence interval (CI) =0.26-0.43] and 47 months (95% CI=0.38-0.56; log-rank, p=0.001) respectively, whereas for group 2 patients treated with other regimens, PFS was 27.48 months (95% CI=0.20-0.33) and OS was 30 months (95% CI=0.21-0.38; log-rank, p=0.001). While none of the patients in group 1 experienced neurotoxicity or other grade 3 or 4 toxicities, 3/38 patients in group 2 experienced grade 3 neutropenia, 4/38 had peripheral sensory neuropathy, 6/38 patients had treatment deferred due to toxicities or allergic reaction to paclitaxel.
CONCLUSION: In the phase III randomized controlled trial combination of ifosfamide and taxanes has shown PFS and OS benefit when compared to single-agent ifosfamide at the expense of significant toxicities. Results from our study show that the combination of CIM is an effective and safe alternative regimen for patients with advanced UCSs. In addition to improved OS and PFS, the main advantage of this regimen over taxane-based regimens includes minimal neuropathy, less use of steroids, and low risk of allergic reaction. CIM should be considered in future prospective studies looking at the treatment of UCS.
Hayslip J, Dressler EV, Weiss H, et al.
Plasma TNF-α and Soluble TNF Receptor Levels after Doxorubicin with or without Co-Administration of Mesna-A Randomized, Cross-Over Clinical Study.
PLoS One. 2015; 10(4):e0124988 [PubMed] Free Access to Full Article Related Publications
Plasma TNF-α and Soluble TNF Receptor Levels after Doxorubicin with or without Co-Administration of Mesna-A Randomized, Cross-Over Clinical Study.
PLoS One. 2015; 10(4):e0124988 [PubMed] Free Access to Full Article Related Publications
PURPOSE: Chemotherapy-induced cognitive impairment (CICI) is a common sequelae of cancer therapy. Recent preclinical observations have suggested that CICI can be mediated by chemotherapy-induced plasma protein oxidation, which triggers TNF-α mediated CNS damage. This study evaluated sodium-2-mercaptoethane sulfonate (Mesna) co-administration with doxorubicin to reduce doxorubicin-induced plasma protein oxidation and resultant cascade of TNF-α, soluble TNF receptor levels and related cytokines.
METHODS: Thirty-two evaluable patients were randomized using a crossover design to receive mesna or saline in either the first or second cycle of doxorubicin in the context of a standard chemotherapy regimen for either non-Hodgkin lymphoma or breast cancer. Mesna (360 mg/m2) or saline administration occurred 15 minutes prior and three hours post doxorubicin. Pre-treatment and post-treatment measurements of oxidative stress, TNF-α and related cytokines were evaluated during the two experimental cycles of chemotherapy.
RESULTS: Co-administration of mesna with chemotherapy reduced post-treatment levels of TNF-related cytokines and TNF-receptor 1 (TNFR1) and TNF-receptor 2 (TNFR2) (p = 0.05 and p = 0.002, respectively). Patients with the highest pre-treatment levels of each cytokine and its receptors were the most likely to benefit from mesna co-administration.
CONCLUSIONS: The extracellular anti-oxidant mesna, when co-administered during a single cycle of doxorubicin, reduced levels of TNF-α and its receptors after that cycle of therapy, demonstrating for the first time a clinical interaction between mesna and doxorubicin, drugs often coincidentally co-administered in multi-agent regimens. These findings support further investigation to determine whether rationally-timed mesna co-administration with redox active chemotherapy may prevent or reduce the cascade of events that lead to CICI.
TRIAL REGISTRATION: clinicaltrials.gov NCT01205503.
METHODS: Thirty-two evaluable patients were randomized using a crossover design to receive mesna or saline in either the first or second cycle of doxorubicin in the context of a standard chemotherapy regimen for either non-Hodgkin lymphoma or breast cancer. Mesna (360 mg/m2) or saline administration occurred 15 minutes prior and three hours post doxorubicin. Pre-treatment and post-treatment measurements of oxidative stress, TNF-α and related cytokines were evaluated during the two experimental cycles of chemotherapy.
RESULTS: Co-administration of mesna with chemotherapy reduced post-treatment levels of TNF-related cytokines and TNF-receptor 1 (TNFR1) and TNF-receptor 2 (TNFR2) (p = 0.05 and p = 0.002, respectively). Patients with the highest pre-treatment levels of each cytokine and its receptors were the most likely to benefit from mesna co-administration.
CONCLUSIONS: The extracellular anti-oxidant mesna, when co-administered during a single cycle of doxorubicin, reduced levels of TNF-α and its receptors after that cycle of therapy, demonstrating for the first time a clinical interaction between mesna and doxorubicin, drugs often coincidentally co-administered in multi-agent regimens. These findings support further investigation to determine whether rationally-timed mesna co-administration with redox active chemotherapy may prevent or reduce the cascade of events that lead to CICI.
TRIAL REGISTRATION: clinicaltrials.gov NCT01205503.
Dobashi A, Goda K, Sumiyama K, et al.
A feasibility study of chemically assisted endoscopic submucosal mechanical dissection using mesna for superficial esophageal squamous cell carcinomas.
Surg Endosc. 2015; 29(11):3373-81 [PubMed] Related Publications
A feasibility study of chemically assisted endoscopic submucosal mechanical dissection using mesna for superficial esophageal squamous cell carcinomas.
Surg Endosc. 2015; 29(11):3373-81 [PubMed] Related Publications
BACKGROUND: Injection of mesna into submucosal layers was recently reported to chemically soften connective tissue and facilitate the gastric endoscopic submucosal dissection (ESD) procedure. This study aimed to evaluate the safety and feasibility of similarly using mesna for esophageal ESD (mesna ESD).
METHODS: We performed mesna ESD in 20 consecutive patients with superficial esophageal squamous cell carcinomas (SESCCs). To do this, a submucosal fluid cushion was initially formed using sodium hyaluronate, and the esophageal lesion was circumferentially isolated with a short blade needle-knife. Mesna solution was then injected into the submucosal layer, which was dissected mechanically by cleavage using the tip of a cap-fitted endoscope. The number of electrosurgical incisions was recorded by computer software in real time. The data from 20 conventional ESD procedures without mesna (consecutive 10 SESCCs pre and post the 20 consecutive mesna ESD) were used for comparison to evaluate the mesna ESD.
RESULTS: The mesna ESDs achieved en bloc and R0 resection success rates of 100 and 95 %, respectively. There was no perforation or uncontrollable hemorrhage during and after mesna ESD, and the median procedural time of submucosal dissection was significantly less with mesna ESD than with conventional ESD (median; 8 vs. 15 min, P < 0.05). There were also significantly fewer electrosurgical incisions made during the mesna ESD than with conventional ESDs (median; 65 vs. 183 times, P < 0.01).
CONCLUSIONS: Mesna ESD for SESCCs is a safe procedure with the potential to facilitate esophageal ESD.
METHODS: We performed mesna ESD in 20 consecutive patients with superficial esophageal squamous cell carcinomas (SESCCs). To do this, a submucosal fluid cushion was initially formed using sodium hyaluronate, and the esophageal lesion was circumferentially isolated with a short blade needle-knife. Mesna solution was then injected into the submucosal layer, which was dissected mechanically by cleavage using the tip of a cap-fitted endoscope. The number of electrosurgical incisions was recorded by computer software in real time. The data from 20 conventional ESD procedures without mesna (consecutive 10 SESCCs pre and post the 20 consecutive mesna ESD) were used for comparison to evaluate the mesna ESD.
RESULTS: The mesna ESDs achieved en bloc and R0 resection success rates of 100 and 95 %, respectively. There was no perforation or uncontrollable hemorrhage during and after mesna ESD, and the median procedural time of submucosal dissection was significantly less with mesna ESD than with conventional ESD (median; 8 vs. 15 min, P < 0.05). There were also significantly fewer electrosurgical incisions made during the mesna ESD than with conventional ESDs (median; 65 vs. 183 times, P < 0.01).
CONCLUSIONS: Mesna ESD for SESCCs is a safe procedure with the potential to facilitate esophageal ESD.
Salman D, Swinden J, Barton S, et al.
Evaluation of the stability profile of anticancer drugs: A review of Ifosfamide and Mesna regimen for the treatment of metastatic soft tissue sarcoma.
J Oncol Pharm Pract. 2016; 22(1):86-91 [PubMed] Related Publications
Evaluation of the stability profile of anticancer drugs: A review of Ifosfamide and Mesna regimen for the treatment of metastatic soft tissue sarcoma.
J Oncol Pharm Pract. 2016; 22(1):86-91 [PubMed] Related Publications
PURPOSE: This paper aims to summarise and critically review the existing published literature with regard to clinical considerations as well as stability testing studies of Ifosfamide and Mesna. It also aims to highlight the factors that should be considered when designing and conducting stability testing experiments.
SUMMARY: Ifosfamide and Mesna are currently given to patients for 14 days continuous home-based infusion for the treatment of soft tissue sarcoma. No previous work has evaluated their stability for more than 7 days under real-life conditions so the current regimen involves patients visiting hospital twice during the 14-day treatment. This may create extra disruption to patients' life style as well as increasing the workload for cancer services.
CONCLUSION: There is a need to conduct stability testing experiments for Ifosfamide and Mesna taking into consideration all of the highlighted factors to mimic standard clinical practice.
SUMMARY: Ifosfamide and Mesna are currently given to patients for 14 days continuous home-based infusion for the treatment of soft tissue sarcoma. No previous work has evaluated their stability for more than 7 days under real-life conditions so the current regimen involves patients visiting hospital twice during the 14-day treatment. This may create extra disruption to patients' life style as well as increasing the workload for cancer services.
CONCLUSION: There is a need to conduct stability testing experiments for Ifosfamide and Mesna taking into consideration all of the highlighted factors to mimic standard clinical practice.
Sumiyama K, Toyoizumi H, Ohya TR, et al.
A double-blind, block-randomized, placebo-controlled trial to identify the chemical assistance effect of mesna submucosal injection for gastric endoscopic submucosal dissection.
Gastrointest Endosc. 2014; 79(5):756-64 [PubMed] Related Publications
A double-blind, block-randomized, placebo-controlled trial to identify the chemical assistance effect of mesna submucosal injection for gastric endoscopic submucosal dissection.
Gastrointest Endosc. 2014; 79(5):756-64 [PubMed] Related Publications
BACKGROUND: Previous animal studies and a pilot clinical trial demonstrated that submucosal injection of a thiol compound called mesna could chemically soften connective tissues and thus facilitate endoscopic submucosal dissection (ESD).
OBJECTIVE: To evaluate whether mesna injection could reduce procedural times for gastric ESD.
DESIGN: Double-blind, block-randomized, controlled trial.
SETTING: University hospital.
PATIENTS: A total of 101 patients with superficial gastric cancer indicated for ESD were enrolled and randomly assigned to either the mesna or control (saline solution) group.
INTERVENTION: Traditional ESD was performed with a single bolus injection of mesna or saline solution.
MAIN OUTCOME MEASUREMENTS: Time for submucosal dissection (TSD).
RESULTS: En bloc resection was achieved for all lesions in the mesna group (53/53) and 51 of 52 lesions (98.08%) in the control group. TSD was not statistically different between the groups (18.62 ± 13.9 [mean ± SD] minutes for the mesna group and 24.58 ± 24.55 [mean ± SD] minutes for the control group; P = .128), and there were fewer time-consuming cases (times over 30 minutes) in the mesna group compared with controls (7/53 vs 15/52; P = .049). Multivariate regression analysis demonstrated that use of mesna, specimen size, and the presence of fibrous scars were significantly correlated with TSD (P < .05).
LIMITATIONS: Single-center study.
CONCLUSION: TSD was not significantly different between the mesna and control injection groups, but multivariate analysis indicated that mesna injection reduced procedural challenges associated with the submucosal dissection. (
CLINICAL TRIAL REGISTRATION NUMBER: UMIN000003786.).
OBJECTIVE: To evaluate whether mesna injection could reduce procedural times for gastric ESD.
DESIGN: Double-blind, block-randomized, controlled trial.
SETTING: University hospital.
PATIENTS: A total of 101 patients with superficial gastric cancer indicated for ESD were enrolled and randomly assigned to either the mesna or control (saline solution) group.
INTERVENTION: Traditional ESD was performed with a single bolus injection of mesna or saline solution.
MAIN OUTCOME MEASUREMENTS: Time for submucosal dissection (TSD).
RESULTS: En bloc resection was achieved for all lesions in the mesna group (53/53) and 51 of 52 lesions (98.08%) in the control group. TSD was not statistically different between the groups (18.62 ± 13.9 [mean ± SD] minutes for the mesna group and 24.58 ± 24.55 [mean ± SD] minutes for the control group; P = .128), and there were fewer time-consuming cases (times over 30 minutes) in the mesna group compared with controls (7/53 vs 15/52; P = .049). Multivariate regression analysis demonstrated that use of mesna, specimen size, and the presence of fibrous scars were significantly correlated with TSD (P < .05).
LIMITATIONS: Single-center study.
CONCLUSION: TSD was not significantly different between the mesna and control injection groups, but multivariate analysis indicated that mesna injection reduced procedural challenges associated with the submucosal dissection. (
CLINICAL TRIAL REGISTRATION NUMBER: UMIN000003786.).
Dorris K, Fouladi M, Davies SM, et al.
Severe allergic reactions to thiol-based cytoprotective agents mesna and amifostine in a child with a supratentorial primitive neuroectodermal tumor.
J Pediatr Hematol Oncol. 2011; 33(6):e250-2 [PubMed] Related Publications
Severe allergic reactions to thiol-based cytoprotective agents mesna and amifostine in a child with a supratentorial primitive neuroectodermal tumor.
J Pediatr Hematol Oncol. 2011; 33(6):e250-2 [PubMed] Related Publications
Both 2-mercaptoethane sulfonate sodium (mesna) and amifostine's active metabolite WR-1065 are thiol-based cytoprotective agents that are critical components of high-dose chemotherapy regimens used to treat various cancers in both adults and children. This case report describes a patient with a supratentorial primitive neuroectodermal tumor who developed severe drug reactions to both mesna and amifostine/WR-1065, suggesting that the thiol component of these agents triggered the adverse reactions. This report highlights the clinical presentation of drug-induced hypersensitivity syndrome in the context of pediatric oncology and the supportive care measures that, if implemented rapidly, may diminish the reaction severity and allow successful completion of chemotherapy.
Sumiyama K, Tajiri H, Gostout CJ, et al.
Chemically assisted submucosal injection facilitates endoscopic submucosal dissection of gastric neoplasms.
Endoscopy. 2010; 42(8):627-32 [PubMed] Related Publications
Chemically assisted submucosal injection facilitates endoscopic submucosal dissection of gastric neoplasms.
Endoscopy. 2010; 42(8):627-32 [PubMed] Related Publications
BACKGROUND AND STUDY AIMS: A randomized in vivo animal study previously demonstrated that topical injection of mesna solution (sodium-2-mercaptoethanesulfonate) chemically softened submucosal connective tissues and facilitated mechanical dissection of the submucosal tissue plane. The present study evaluated the technical feasibility and safety of chemically assisted endoscopic submucosal dissection (CA-ESD) using mesna in 20 consecutive patients who underwent endoscopic excision of gastric neoplasm.
MATERIALS AND METHODS: Following the margination of the lesion with a mucosal circumcision, 4 - 12 mL of 10 % mesna solution was injected into the submucosal layer. Mechanical submucosal dissection was then performed by bluntly cleaving the chemically treated submucosal layer with the tip of a cap-fitted gastroscope. The use of cautery was restricted to prophylactic hemostasis, dissection of the coagulated vessels and persistent submucosal tissues, and the final snare resection. Post-therapeutic ulceration repair and adverse events were followed up during a 1-week hospitalization and by repeat endoscopies at 1 day, 1 week, and 1 month after the procedure.
RESULTS: Sixteen gastric cancers and four adenomas were treated in this study. The sampled tissue measured 38.25 +/- 14.53 mm, with an en bloc resection rate of 100 %. Mean operation time was 21.17 +/- 11.6 minutes. The time spent using cautery was limited to 26.1 % of the total submucosal dissection time. Ulcerations healed normally without complications.
CONCLUSIONS: This preliminary study demonstrates that submucosal injection of mesna facilitates and expedites mechanical submucosal dissection. The major limitations in this study include the single-arm study design and a small patient population.
MATERIALS AND METHODS: Following the margination of the lesion with a mucosal circumcision, 4 - 12 mL of 10 % mesna solution was injected into the submucosal layer. Mechanical submucosal dissection was then performed by bluntly cleaving the chemically treated submucosal layer with the tip of a cap-fitted gastroscope. The use of cautery was restricted to prophylactic hemostasis, dissection of the coagulated vessels and persistent submucosal tissues, and the final snare resection. Post-therapeutic ulceration repair and adverse events were followed up during a 1-week hospitalization and by repeat endoscopies at 1 day, 1 week, and 1 month after the procedure.
RESULTS: Sixteen gastric cancers and four adenomas were treated in this study. The sampled tissue measured 38.25 +/- 14.53 mm, with an en bloc resection rate of 100 %. Mean operation time was 21.17 +/- 11.6 minutes. The time spent using cautery was limited to 26.1 % of the total submucosal dissection time. Ulcerations healed normally without complications.
CONCLUSIONS: This preliminary study demonstrates that submucosal injection of mesna facilitates and expedites mechanical submucosal dissection. The major limitations in this study include the single-arm study design and a small patient population.
Masuda N, Negoro S, Hausheer F, et al.
Phase I and pharmacologic study of BNP7787, a novel chemoprotector in patients with advanced non-small cell lung cancer.
Cancer Chemother Pharmacol. 2011; 67(3):533-42 [PubMed] Free Access to Full Article Related Publications
Phase I and pharmacologic study of BNP7787, a novel chemoprotector in patients with advanced non-small cell lung cancer.
Cancer Chemother Pharmacol. 2011; 67(3):533-42 [PubMed] Free Access to Full Article Related Publications
PURPOSE: We conducted a phase I trial of BNP7787 (disodium 2,2'-dithio-bis-ethane sulfonate, Tavocept™), a novel chemoprotective and antitumor enhancing agent administered in combination with paclitaxel and cisplatin. The primary aim was to determine a safe and potentially efficacious BNP7787 dose for preventing and mitigating paclitaxel- and cisplatin-induced toxicities and to evaluate for preliminary evidence of efficacy of treatment.
PATIENTS AND METHODS: Twenty-two patients with stage IIIB/IV non-small cell lung cancer (NSCLC) received BNP7787 alone 1 week before co-administration of BNP7787 with paclitaxel followed by cisplatin. Twenty-one patients were treated with BNP7787 in escalating doses of 4.1-41.0 g/m² concurrently with paclitaxel 175 mg/m² and cisplatin 75 mg/m² every 3 weeks.
RESULTS: The appropriate dose was determined to be 18.4 g/m² of BNP7787 although no dose-limiting toxicity was observed up to 41.0 g/m². Mild intravenous site discomfort, thirst, and nausea were the most common toxicities. One patient developed grade 2 skin rash, which was severe enough to preclude further study treatment. The AUC(0-inf) of the metabolite mesna was approximately 6.3% of the AUC(0-inf) of BNP7787. Co-administration of paclitaxel and cisplatin did not appear to influence the pharmacokinetics of BNP7787 and mesna. The overall response rate was encouraging; 43% including 11 patients with prior chemotherapy.
CONCLUSIONS: The recommended dose for phase II/III studies is 18.4 mg/m² of BNP7787 in combination with paclitaxel and cisplatin. Further studies are warranted to assess whether BNP7787 prevents and mitigates common and serious paclitaxel- and cisplatin-related side effects and enhances the efficacy of paclitaxel and cisplatin in advanced NSCLC patients.
PATIENTS AND METHODS: Twenty-two patients with stage IIIB/IV non-small cell lung cancer (NSCLC) received BNP7787 alone 1 week before co-administration of BNP7787 with paclitaxel followed by cisplatin. Twenty-one patients were treated with BNP7787 in escalating doses of 4.1-41.0 g/m² concurrently with paclitaxel 175 mg/m² and cisplatin 75 mg/m² every 3 weeks.
RESULTS: The appropriate dose was determined to be 18.4 g/m² of BNP7787 although no dose-limiting toxicity was observed up to 41.0 g/m². Mild intravenous site discomfort, thirst, and nausea were the most common toxicities. One patient developed grade 2 skin rash, which was severe enough to preclude further study treatment. The AUC(0-inf) of the metabolite mesna was approximately 6.3% of the AUC(0-inf) of BNP7787. Co-administration of paclitaxel and cisplatin did not appear to influence the pharmacokinetics of BNP7787 and mesna. The overall response rate was encouraging; 43% including 11 patients with prior chemotherapy.
CONCLUSIONS: The recommended dose for phase II/III studies is 18.4 mg/m² of BNP7787 in combination with paclitaxel and cisplatin. Further studies are warranted to assess whether BNP7787 prevents and mitigates common and serious paclitaxel- and cisplatin-related side effects and enhances the efficacy of paclitaxel and cisplatin in advanced NSCLC patients.
Vlahovic A, Simic R, Djokic D, Ceran C
Diffuse neonatal hemangiomatosis treatment with cyclophosphamide: a case report.
J Pediatr Hematol Oncol. 2009; 31(11):858-60 [PubMed] Related Publications
Diffuse neonatal hemangiomatosis treatment with cyclophosphamide: a case report.
J Pediatr Hematol Oncol. 2009; 31(11):858-60 [PubMed] Related Publications
We present 3-month-old male infant with diffuse neonatal hemangiomatosis. There were 63 cutaneous hemangiomas over the scalp, face, trunk, and extremities. Computed tomography scan revealed the presence of hemangiomas in the liver and kidneys; laryngobronchoscopy identified the presence of hemangioma in tracheobronchial tree. The child had symptoms of heart failure therefore digitals and diuretics were administrated. Thyroid functions were normal. Treatment with corticosteroids, in dose of 3 mg/kg/d intravenously, was initiated. As there was no significant clinical improvement, cyclophosphamide was administrated. He received 4 courses, 10 days apart. Each course consisted of 10 mg/kg/d of cyclophosphamide and 10 mg/kg/d of mesna for 4 consecutive days. After 4 cycles of cyclophosphamide, the liver was notably decreased in size and the cardiac failure was resolved. Magnetic resonance imaging of the abdomen revealed the marked decrease in size of the liver hemangioma. After 3 years of follow-up the child is well developed, fully recovered, without cardiologic or respiratory problems.
Hensley ML, Hagerty KL, Kewalramani T, et al.
American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants.
J Clin Oncol. 2009; 27(1):127-45 [PubMed] Related Publications
American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants.
J Clin Oncol. 2009; 27(1):127-45 [PubMed] Related Publications
PURPOSE: To update a clinical practice guideline on the use of chemotherapy and radiation therapy protectants for patients with cancer.
METHODS: An update committee reviewed literature published since the last guideline update in 2002.
RESULTS: Thirty-nine reports met the inclusion criteria: palifermin and dexrazoxane, three reports (two studies) each; amifostine, 33 reports (31 studies); and mesna, no published randomized trials identified since 2002.
RECOMMENDATIONS: Dexrazoxane is not recommended for routine use in breast cancer (BC) in adjuvant setting, or metastatic setting with initial doxorubicin-based chemotherapy. Consider use with metastatic BC and other malignancies, for patients who have received more than 300 mg/m(2) doxorubicin who may benefit from continued doxorubicin-containing therapy. Cardiac monitoring should continue in patients receiving doxorubicin. Amifostine may be considered for prevention of cisplatin-associated nephrotoxicity, reduction of grade 3 to 4 neutropenia (alternative strategies are reasonable), and to decrease acute and late xerostomia with fractionated radiation therapy alone for head and neck cancer. It is not recommended for protection against thrombocytopenia, prevention of platinum-associated neurotoxicity or ototoxicity or paclitaxel-associated neuropathy, prevention of radiation therapy-associated mucositis in head and neck cancer, or prevention of esophagitis during concurrent chemoradiotherapy for non-small-cell lung cancer. Palifermin is recommended to decrease severe mucositis in autologous stem-cell transplantation (SCT) for hematologic malignancies with total-body irradiation (TBI) conditioning regimens, and considered for patients undergoing myeloablative allogeneic SCT with TBI-based conditioning regimens. Data are insufficient to recommend use in the non-SCT setting.
METHODS: An update committee reviewed literature published since the last guideline update in 2002.
RESULTS: Thirty-nine reports met the inclusion criteria: palifermin and dexrazoxane, three reports (two studies) each; amifostine, 33 reports (31 studies); and mesna, no published randomized trials identified since 2002.
RECOMMENDATIONS: Dexrazoxane is not recommended for routine use in breast cancer (BC) in adjuvant setting, or metastatic setting with initial doxorubicin-based chemotherapy. Consider use with metastatic BC and other malignancies, for patients who have received more than 300 mg/m(2) doxorubicin who may benefit from continued doxorubicin-containing therapy. Cardiac monitoring should continue in patients receiving doxorubicin. Amifostine may be considered for prevention of cisplatin-associated nephrotoxicity, reduction of grade 3 to 4 neutropenia (alternative strategies are reasonable), and to decrease acute and late xerostomia with fractionated radiation therapy alone for head and neck cancer. It is not recommended for protection against thrombocytopenia, prevention of platinum-associated neurotoxicity or ototoxicity or paclitaxel-associated neuropathy, prevention of radiation therapy-associated mucositis in head and neck cancer, or prevention of esophagitis during concurrent chemoradiotherapy for non-small-cell lung cancer. Palifermin is recommended to decrease severe mucositis in autologous stem-cell transplantation (SCT) for hematologic malignancies with total-body irradiation (TBI) conditioning regimens, and considered for patients undergoing myeloablative allogeneic SCT with TBI-based conditioning regimens. Data are insufficient to recommend use in the non-SCT setting.
Miller AA, Wang XF, Gu L, et al.
Phase II randomized study of dose-dense docetaxel and cisplatin every 2 weeks with pegfilgrastim and darbepoetin alfa with and without the chemoprotector BNP7787 in patients with advanced non-small cell lung cancer (CALGB 30303).
J Thorac Oncol. 2008; 3(10):1159-65 [PubMed] Related Publications
Phase II randomized study of dose-dense docetaxel and cisplatin every 2 weeks with pegfilgrastim and darbepoetin alfa with and without the chemoprotector BNP7787 in patients with advanced non-small cell lung cancer (CALGB 30303).
J Thorac Oncol. 2008; 3(10):1159-65 [PubMed] Related Publications
INTRODUCTION: We investigated dose-dense docetaxel and cisplatin in patients with measurable non-small cell lung cancer in a randomized phase II study without [A] or with [B] a putative chemoprotective agent, BNP7787.
PATIENTS AND METHODS: Chemotherapy-naive patients with stage IIIB (effusion) or IV, performance status 0 to 1, and adequate organ function were eligible. Treatment with docetaxel 75 mg/m followed by cisplatin 75 mg/m over 1 hour day 1 with darbepoetin 200 mug day 1 and pegfilgrastim 6 mg day 2 without/with BNP7787 before cisplatin was repeated every other week for up to 6 cycles. The primary end point was to differentiate between grade >/=2 neurotoxicity rates of 30% on [A] and 10% on [B]. Feasibility was prospectively defined as febrile neutropenia in <10% of patients and RESULTS: Of 160 patients enrolled, 5 never started therapy and 4 were ineligible. Neurotoxicity grade >/=2 occurred in 32% on [A] and 29% on [B]. The incidence of febrile neutropenia was 4% on [A] and 3% on [B]. Treatment delays occurred in 13% and 20% of patients on [A] and [B], respectively. Completion rates for 3/6 cycles were 84%/51% on [A] and 84%/53% on [B]. Objective response rates were 55% on [A] and 51% on [B]. Median progression-free/overall survival times were 5.5/10.7 on [A] and 6.5/14.1 month on [B].
CONCLUSIONS: This dose-dense treatment regimen is active, feasible, and tolerable. Its further investigation in the curative setting in non-small cell lung cancer should be considered. BNP7787 did not result in significant protection from neurotoxicity.
PATIENTS AND METHODS: Chemotherapy-naive patients with stage IIIB (effusion) or IV, performance status 0 to 1, and adequate organ function were eligible. Treatment with docetaxel 75 mg/m followed by cisplatin 75 mg/m over 1 hour day 1 with darbepoetin 200 mug day 1 and pegfilgrastim 6 mg day 2 without/with BNP7787 before cisplatin was repeated every other week for up to 6 cycles. The primary end point was to differentiate between grade >/=2 neurotoxicity rates of 30% on [A] and 10% on [B]. Feasibility was prospectively defined as febrile neutropenia in <10% of patients and RESULTS: Of 160 patients enrolled, 5 never started therapy and 4 were ineligible. Neurotoxicity grade >/=2 occurred in 32% on [A] and 29% on [B]. The incidence of febrile neutropenia was 4% on [A] and 3% on [B]. Treatment delays occurred in 13% and 20% of patients on [A] and [B], respectively. Completion rates for 3/6 cycles were 84%/51% on [A] and 84%/53% on [B]. Objective response rates were 55% on [A] and 51% on [B]. Median progression-free/overall survival times were 5.5/10.7 on [A] and 6.5/14.1 month on [B].
CONCLUSIONS: This dose-dense treatment regimen is active, feasible, and tolerable. Its further investigation in the curative setting in non-small cell lung cancer should be considered. BNP7787 did not result in significant protection from neurotoxicity.
Wolfson AH, Brady MF, Rocereto T, et al.
A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus.
Gynecol Oncol. 2007; 107(2):177-85 [PubMed] Free Access to Full Article Related Publications
A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus.
Gynecol Oncol. 2007; 107(2):177-85 [PubMed] Free Access to Full Article Related Publications
PURPOSE: After initial surgery, there has been no established consensus regarding adjunctive therapy for patients with uterine carcinosarcoma (CS). This study was designed to compare patient outcome following treatment with adjuvant whole abdominal irradiation (WAI) versus (vs.) chemotherapy for patients with this rare group of female pelvic malignancies.
PATIENTS AND METHODS: Eligible, consenting women with stage I-IV uterine CS, no more than 1 cm postsurgical residuum and/or no extra-abdominal spread had their treatments randomly assigned as either WAI or three cycles of cisplatin (C), ifosfamide (I), and mesna (M).
RESULTS: 232 patients were enrolled, of whom 206 (WAI=105; CIM=101) were deemed eligible. Patient demographics and characteristics were similar between arms. FIGO stage (both arms) was: I=64 (31%); II=26 (13%); III=92 (45%); IV=24 (12%). The estimated crude probability of recurring within 5 years was 58% (WAI) and 52% (CIM). Adjusting for stage and age, the recurrence rate was 21% lower for CIM patients than for WAI patients (relative hazard [RH]=0.789, 95% confidence interval [CI]: (0.530-1.176), p=0.245, 2-tail test). The estimated death rate was 29% lower among the CIM group (RH=0.712, 95% CI: 0.484-1.048, p=0.085, two-tail test).
CONCLUSION: We did not find a statistically significant advantage in recurrence rate or survival for adjuvant CIM over WAI in patients with uterine CS. However, the observed differences favor the use of combination chemotherapy in future trials.
PATIENTS AND METHODS: Eligible, consenting women with stage I-IV uterine CS, no more than 1 cm postsurgical residuum and/or no extra-abdominal spread had their treatments randomly assigned as either WAI or three cycles of cisplatin (C), ifosfamide (I), and mesna (M).
RESULTS: 232 patients were enrolled, of whom 206 (WAI=105; CIM=101) were deemed eligible. Patient demographics and characteristics were similar between arms. FIGO stage (both arms) was: I=64 (31%); II=26 (13%); III=92 (45%); IV=24 (12%). The estimated crude probability of recurring within 5 years was 58% (WAI) and 52% (CIM). Adjusting for stage and age, the recurrence rate was 21% lower for CIM patients than for WAI patients (relative hazard [RH]=0.789, 95% confidence interval [CI]: (0.530-1.176), p=0.245, 2-tail test). The estimated death rate was 29% lower among the CIM group (RH=0.712, 95% CI: 0.484-1.048, p=0.085, two-tail test).
CONCLUSION: We did not find a statistically significant advantage in recurrence rate or survival for adjuvant CIM over WAI in patients with uterine CS. However, the observed differences favor the use of combination chemotherapy in future trials.
Hogle WP
Cytoprotective agents used in the treatment of patients with cancer.
Semin Oncol Nurs. 2007; 23(3):213-24 [PubMed] Related Publications
Cytoprotective agents used in the treatment of patients with cancer.
Semin Oncol Nurs. 2007; 23(3):213-24 [PubMed] Related Publications
OBJECTIVE: To provide a review of the literature of commonly prescribed cytoprotective agents used in the treatment of patients with cancer.
DATA SOURCES: Journal articles, research reports, review articles, and web sites.
CONCLUSION: Multiple agents have been theorized to have cytoprotective properties. Significant evidence exists supporting the use of some cytoprotective agents approved by the US Food and Drug Administration (FDA). More research is needed to determine the efficacy of new cytoprotective agents and expanded indications for those agents currently used.
IMPLICATIONS FOR NURSING PRACTICE: Knowledge of the indications for and side effect profiles of cytoprotective agents is a necessary component of oncology nursing care. Familiarity with evidence-based research that supports or refutes the use of FDA-approved cytoprotective agents or alternative agents is helpful when suggesting, prescribing, or administering such agents.
DATA SOURCES: Journal articles, research reports, review articles, and web sites.
CONCLUSION: Multiple agents have been theorized to have cytoprotective properties. Significant evidence exists supporting the use of some cytoprotective agents approved by the US Food and Drug Administration (FDA). More research is needed to determine the efficacy of new cytoprotective agents and expanded indications for those agents currently used.
IMPLICATIONS FOR NURSING PRACTICE: Knowledge of the indications for and side effect profiles of cytoprotective agents is a necessary component of oncology nursing care. Familiarity with evidence-based research that supports or refutes the use of FDA-approved cytoprotective agents or alternative agents is helpful when suggesting, prescribing, or administering such agents.
Khaw SL, Downie PA, Waters KD, et al.
Adverse hypersensitivity reactions to mesna as adjunctive therapy for cyclophosphamide.
Pediatr Blood Cancer. 2007; 49(3):341-3 [PubMed] Related Publications
Adverse hypersensitivity reactions to mesna as adjunctive therapy for cyclophosphamide.
Pediatr Blood Cancer. 2007; 49(3):341-3 [PubMed] Related Publications
Mesna is widely used for the prevention of cyclophosphamide-related hemorrhagic cystitis. It has been associated with hypersensitivity-like cutaneous and systemic reactions in adult patients. We report a series of children with malignant disease, who developed such reactions following mesna administration and discuss possible mechanisms and management issues.
Soff GA, Wang H, Cundiff DL, et al.
In vivo generation of angiostatin isoforms by administration of a plasminogen activator and a free sulfhydryl donor: a phase I study of an angiostatic cocktail of tissue plasminogen activator and mesna.
Clin Cancer Res. 2005; 11(17):6218-25 [PubMed] Related Publications
In vivo generation of angiostatin isoforms by administration of a plasminogen activator and a free sulfhydryl donor: a phase I study of an angiostatic cocktail of tissue plasminogen activator and mesna.
Clin Cancer Res. 2005; 11(17):6218-25 [PubMed] Related Publications
PURPOSE: Angiostatin4.5 (AS4.5), the endogenous human angiostatin, is derived from plasminogen in a two-step process. A plasminogen activator converts plasminogen to plasmin, then plasmin undergoes autoproteolysis to AS4.5. A free sulfhydryl donor can mediate plasmin autoproteolysis. To translate this process to human cancer therapy, we conducted a phase I trial of administration of a tissue plasminogen activator (tPA) with a free sulfhydryl donor (mesna).
PATIENTS AND METHODS: Fifteen patients with advanced solid tumors were treated. The dose of tPA was escalated (cohorts; 1, 2, 3, 5, and 7.5 mg/h for 6 hours). Mesna was administered as a 240 mg/m2 bolus followed by an infusion of 50 mg/h, concurrent with tPA. Both tPA and mesna were administered 3 consecutive days every 14 days.
RESULTS: No dose-limiting toxicity was observed. Two AS4.5 isoforms were generated, Lys-AS4.5 and Glu-AS4.5. Mean baseline Lys-AS4.5 level was 20.4 nmol/L (SE, 2.9). In the 5 mg/h tPA cohort, Lys-AS4.5 levels increased by an average of 143% or 24 nmol/L (SE, 4.9) above baseline. Glu-AS4.5 (M(r) approximately 62,000) was also generated (additional 77 amino acids at amino terminus compared with Lys-AS4.5). Glu-AS4.5 level at baseline was undetectable in four of five patients in the 5 mg/h tPA cohort, but at end of infusion, was approximately 67 nmol/L (SE, 20). Two patients in the 5 mg/h tPA cohort experienced decreases in tumor markers with treatment, although no clinical objective responses were observed.
CONCLUSION: This study shows that in vivo generation of AS4.5 is safe in humans and may provide a practical approach to achieve antiangiogenic therapy.
PATIENTS AND METHODS: Fifteen patients with advanced solid tumors were treated. The dose of tPA was escalated (cohorts; 1, 2, 3, 5, and 7.5 mg/h for 6 hours). Mesna was administered as a 240 mg/m2 bolus followed by an infusion of 50 mg/h, concurrent with tPA. Both tPA and mesna were administered 3 consecutive days every 14 days.
RESULTS: No dose-limiting toxicity was observed. Two AS4.5 isoforms were generated, Lys-AS4.5 and Glu-AS4.5. Mean baseline Lys-AS4.5 level was 20.4 nmol/L (SE, 2.9). In the 5 mg/h tPA cohort, Lys-AS4.5 levels increased by an average of 143% or 24 nmol/L (SE, 4.9) above baseline. Glu-AS4.5 (M(r) approximately 62,000) was also generated (additional 77 amino acids at amino terminus compared with Lys-AS4.5). Glu-AS4.5 level at baseline was undetectable in four of five patients in the 5 mg/h tPA cohort, but at end of infusion, was approximately 67 nmol/L (SE, 20). Two patients in the 5 mg/h tPA cohort experienced decreases in tumor markers with treatment, although no clinical objective responses were observed.
CONCLUSION: This study shows that in vivo generation of AS4.5 is safe in humans and may provide a practical approach to achieve antiangiogenic therapy.
Olver I, Keefe D, Myers M, Caruso D
A phase I study of prolonged ambulatory infusion of Ifosfamide with oral mesna.
Chemotherapy. 2005; 51(2-3):142-6 [PubMed] Related Publications
A phase I study of prolonged ambulatory infusion of Ifosfamide with oral mesna.
Chemotherapy. 2005; 51(2-3):142-6 [PubMed] Related Publications
BACKGROUND: Oral mesna allows investigation of ifosfamide as a prolonged ambulatory infusion for dose-intense out-patient use.
METHODS: Cohorts of 3 patients received escalating doses of ifosfamide from 200 to 1,000 mg/m2/day as continuous ambulatory infusion with oral mesna at 30% of the ifosfamide dose every 6 h commencing 2 h prior to ifosfamide infusion as uroprotection on a 14-day schedule with cycles repeated every 28 days.
RESULTS: Fifteen patients received a median of three cycles. Dose-limiting toxicities with cycle 1 were lethargy and hepatotoxicity at 1,000 mg/m2/14 days. Transient transaminase elevation was seen at all dose levels. The other grade 3 toxicities were single episodes of anaemia, granulocytopenia, nausea and hypotension. The best response was stable disease in a patient with thyroid cancer.
CONCLUSION: Ambulatory infusion of 600 mg/m2 ifosfamide with 180 mg/m2 oral mesna was considered suitable for phase II trials and delivers dose-intense out-patient therapy without urotoxicity.
METHODS: Cohorts of 3 patients received escalating doses of ifosfamide from 200 to 1,000 mg/m2/day as continuous ambulatory infusion with oral mesna at 30% of the ifosfamide dose every 6 h commencing 2 h prior to ifosfamide infusion as uroprotection on a 14-day schedule with cycles repeated every 28 days.
RESULTS: Fifteen patients received a median of three cycles. Dose-limiting toxicities with cycle 1 were lethargy and hepatotoxicity at 1,000 mg/m2/14 days. Transient transaminase elevation was seen at all dose levels. The other grade 3 toxicities were single episodes of anaemia, granulocytopenia, nausea and hypotension. The best response was stable disease in a patient with thyroid cancer.
CONCLUSION: Ambulatory infusion of 600 mg/m2 ifosfamide with 180 mg/m2 oral mesna was considered suitable for phase II trials and delivers dose-intense out-patient therapy without urotoxicity.
Boven E, Westerman M, van Groeningen CJ, et al.
Phase I and pharmacokinetic study of the novel chemoprotector BNP7787 in combination with cisplatin and attempt to eliminate the hydration schedule.
Br J Cancer. 2005; 92(9):1636-43 [PubMed] Free Access to Full Article Related Publications
Phase I and pharmacokinetic study of the novel chemoprotector BNP7787 in combination with cisplatin and attempt to eliminate the hydration schedule.
Br J Cancer. 2005; 92(9):1636-43 [PubMed] Free Access to Full Article Related Publications
BNP7787 (disodium 2,2'-dithio-bis-ethane sulphonate; Tavocept) is a novel agent developed to protect against cisplatin (cis-diammine-dichloroplatinum(II))-associated chronic toxicities. In this study, we determined the recommended dose of BNP7787 when preceding a fixed dose of cisplatin, the pharmacokinetics (PKs) and the possible reduction of saline hydration. Patients with advanced solid tumours received BNP7787 in escalating doses of 4.1-41 g m(-2) as a 15-min intravenous (i.v.) infusion followed by cisplatin 75 mg m(-2) as a 60-min i.v. infusion together with pre- and postcisplatin saline hydration in a volume of 2200 ml; cycles were repeated every 3 weeks. PK was carried out using BNP7787, cisplatin and the combination. Twenty-five patients were enrolled in stage I of the study to determine the recommended dose of BNP7787. No dose-limiting toxicity was reached. The highest dose level of 41 g m(-2) resulted in a low incidence of grade 2 toxicities, being nausea and vomiting, dry mouth or bad taste and i.v. injection site discomfort. Doses of BNP7787 > or = 18.4 g m(-2) did not show a drug interaction between BNP7787 and cisplatin. In stage II of the study, patients received a fixed dose of BNP7787 of 18.4 g m(-2) preceding cisplatin and were entered in prespecified reduced saline hydration steps. A total of 21 patients in cohorts of six to nine patients received reduced saline hydration of 1600 ml (step A), 1000 ml (step B) and 500 ml (step C). In step C, two out of six evaluable patients experienced grade 1 nephrotoxicity. Cisplatin acute toxicities in all 46 patients were as expected. Only five patients complained of paresthesias grade 1 and six developed slight audiometric changes. Partial tumour response was observed in four patients and stable disease in 15 patients. In conclusion, BNP7787 was tolerated well up to doses of 41 g m(-2). The recommended dose of 18.4 g m(-2) enabled safe reduction of the saline hydration schedule for cisplatin to 1000 ml. Further studies will assess whether BNP7787 offers protection against platinum-related late side effects.
Goren MP, Epelman S, Bush DA
Urine mesna excretion after intravenous and oral dosing in ifosfamide-treated children.
Cancer Chemother Pharmacol. 2004; 54(3):237-40 [PubMed] Related Publications
Urine mesna excretion after intravenous and oral dosing in ifosfamide-treated children.
Cancer Chemother Pharmacol. 2004; 54(3):237-40 [PubMed] Related Publications
PURPOSE: To describe mesna excretion in children.
PATIENTS AND METHODS: We studied 14 children (aged 1-18 years) who received 1.8 g/m(2) of ifosfamide per day for 5 days. For uroprotection, the children were given intravenous mesna (equal to 20% of the ifosfamide dose) followed by two oral doses (each equal to 40% of the ifosfamide dose). The concentrations of mesna and the metabolite dimesna were measured in urine samples collected on treatment days 1 and 5.
RESULTS: Of 14 patients enrolled, 11 (aged 4-18 years) were evaluable. The profiles of mesna excretion rates were similar on days 1 and 5. Mesna excretion declined rapidly over 1-2 h after intravenous dosing. Increases in mesna excretion after oral dosing lagged by 2-4 h. About 21% of the mesna administered was excreted unchanged over 24 h on both days 1 and 5. The proportion excreted varied by severalfold between patients, but there was no association with age.
CONCLUSION: The profile of mesna excretion after intravenous and oral dosing in these children was similar to that in reported studies of ifosfamide-treated adults.
PATIENTS AND METHODS: We studied 14 children (aged 1-18 years) who received 1.8 g/m(2) of ifosfamide per day for 5 days. For uroprotection, the children were given intravenous mesna (equal to 20% of the ifosfamide dose) followed by two oral doses (each equal to 40% of the ifosfamide dose). The concentrations of mesna and the metabolite dimesna were measured in urine samples collected on treatment days 1 and 5.
RESULTS: Of 14 patients enrolled, 11 (aged 4-18 years) were evaluable. The profiles of mesna excretion rates were similar on days 1 and 5. Mesna excretion declined rapidly over 1-2 h after intravenous dosing. Increases in mesna excretion after oral dosing lagged by 2-4 h. About 21% of the mesna administered was excreted unchanged over 24 h on both days 1 and 5. The proportion excreted varied by severalfold between patients, but there was no association with age.
CONCLUSION: The profile of mesna excretion after intravenous and oral dosing in these children was similar to that in reported studies of ifosfamide-treated adults.
Verschraagen M, Boven E, Torun E, et al.
Pharmacokinetic behaviour of the chemoprotectants BNP7787 and mesna after an i.v. bolus injection in rats.
Br J Cancer. 2004; 90(8):1654-9 [PubMed] Free Access to Full Article Related Publications
Pharmacokinetic behaviour of the chemoprotectants BNP7787 and mesna after an i.v. bolus injection in rats.
Br J Cancer. 2004; 90(8):1654-9 [PubMed] Free Access to Full Article Related Publications
In preclinical studies, BNP7787 (disodium 2,2'-dithio-bis-ethane sulphonate), the disulphide form of mesna, has demonstrated selective protection against cisplatin-induced nephrotoxicity due to conversion into mesna inactivating toxic platinum species. Mesna (sodium 2-mercapto ethane sulphonate), however, can affect the antitumour activity of cisplatin, while BNP7787 does not interfere with the antitumour activity. To understand the difference in interference with cisplatin-induced antitumour activity between BNP7787 and mesna as well to characterise the selective nephroprotection by BNP7787, the pharmacokinetics of BNP7787 and mesna, each given i.v. 1000 mg x kg(-1), were determined in plasma, kidney, liver, red blood cells (RBC), skeletal muscle and tumour of Fischer rats bearing subcutaneously implanted WARD colon tumours. The following results were obtained: (1). high concentrations of BNP7787 and mesna were observed in the plasma and kidney after administration of BNP7787 or mesna, except for mesna in plasma after BNP7787 administration; (2). in all other sampled compartments, the AUC values of both compounds were at least 5.5-fold lower than the corresponding values in kidney; (3). the AUC of mesna in plasma after mesna administration was comparable to the AUC of mesna in kidney after a dose of BNP7787 that can completely prevent cisplatin-induced nephrotoxicity in rats; (4). the AUC of mesna in plasma was five-fold higher relative to the AUC of mesna following BNP7787 administration (P<0.01). In conclusion, the five-fold higher AUC of mesna in plasma after mesna administration and the fact that mesna is more reactive with (hydrated) cisplatin than its disulphide form BNP7787 represent a plausible explanation as to why mesna administration can reduce the antitumour activity of cisplatin. After BNP7787 administration, the distribution of BNP7787 and mesna was restricted to the kidney, which confirmed the selective protection of the kidney by BNP7787.
Mace JR, Keohan ML, Bernardy H, et al.
Crossover randomized comparison of intravenous versus intravenous/oral mesna in soft tissue sarcoma treated with high-dose ifosfamide.
Clin Cancer Res. 2003; 9(16 Pt 1):5829-34 [PubMed] Related Publications
Crossover randomized comparison of intravenous versus intravenous/oral mesna in soft tissue sarcoma treated with high-dose ifosfamide.
Clin Cancer Res. 2003; 9(16 Pt 1):5829-34 [PubMed] Related Publications
PURPOSE: We conducted our study to determine the pharmacokinetics (PK) and clinical efficacy of oral mesna in patients receiving ifosfamide for soft tissue sarcoma.
EXPERIMENTAL DESIGN: Seventeen patients were enrolled in a randomized prospective Phase I/II study. Seventeen patients were exposed to study medication. Ifosfamide was given at a dose of 2 g/m2/day for 5 days on a 21-day cycle. Before the first cycle, all patients were randomized onto a crossover design and received either the approved i.v. or i.v./oral mesna regimen, with crossover for the second cycle of chemotherapy. The i.v. mesna regimen consisted of dosings (20% ifosfamide dose) at 0, 4, and 8 h. The i.v./oral arm consisted of an i.v. mesna dosing (20% ifosfamide dose) at 0 h, followed by oral tablet dosing (40% ifosfamide dose) at 2 and 6 h. In-patient clinical monitoring and phlebotomy and urine sampling for mesna, dimesna, and ifosfamide PK were performed on all chemotherapy days.
RESULTS: Thirteen patients were evaluable for PK and 17 for efficacy and toxicity. No significant differences were detected in the plasma PK of the concomitantly infused ifosfamide. Rates of hemorrhagic cystitis were similar across mesna schedules. Four of 10 evaluable patients demonstrated objective response.
CONCLUSION: On the basis of our study, an i.v./oral mesna regimen is at least as uroprotective as the approved i.v. regimen. The i.v./oral regimen will improve patient tolerance and convenience, allow for a reduction in elective hospitalizations for ifosfamide chemotherapy, reduce the potential morbidity associated with inpatient administration of chemotherapy, and likely result in decreased costs of care.
EXPERIMENTAL DESIGN: Seventeen patients were enrolled in a randomized prospective Phase I/II study. Seventeen patients were exposed to study medication. Ifosfamide was given at a dose of 2 g/m2/day for 5 days on a 21-day cycle. Before the first cycle, all patients were randomized onto a crossover design and received either the approved i.v. or i.v./oral mesna regimen, with crossover for the second cycle of chemotherapy. The i.v. mesna regimen consisted of dosings (20% ifosfamide dose) at 0, 4, and 8 h. The i.v./oral arm consisted of an i.v. mesna dosing (20% ifosfamide dose) at 0 h, followed by oral tablet dosing (40% ifosfamide dose) at 2 and 6 h. In-patient clinical monitoring and phlebotomy and urine sampling for mesna, dimesna, and ifosfamide PK were performed on all chemotherapy days.
RESULTS: Thirteen patients were evaluable for PK and 17 for efficacy and toxicity. No significant differences were detected in the plasma PK of the concomitantly infused ifosfamide. Rates of hemorrhagic cystitis were similar across mesna schedules. Four of 10 evaluable patients demonstrated objective response.
CONCLUSION: On the basis of our study, an i.v./oral mesna regimen is at least as uroprotective as the approved i.v. regimen. The i.v./oral regimen will improve patient tolerance and convenience, allow for a reduction in elective hospitalizations for ifosfamide chemotherapy, reduce the potential morbidity associated with inpatient administration of chemotherapy, and likely result in decreased costs of care.
Smith PF, Booker BM, Creaven P, et al.
Pharmacokinetics and pharmacodynamics of mesna-mediated plasma cysteine depletion.
J Clin Pharmacol. 2003; 43(12):1324-8 [PubMed] Related Publications
Pharmacokinetics and pharmacodynamics of mesna-mediated plasma cysteine depletion.
J Clin Pharmacol. 2003; 43(12):1324-8 [PubMed] Related Publications
Cellular glutathione (GSH) levels are related to the resistance of tumor cells to platinum and alkylating agents, and depletion of GSH may enhance the activity of these drugs. The pharmacodynamic effects of mesna on depleting plasma cysteine, a GSH precursor, were evaluated in 22 patients as part of a Phase I study. Escalating doses of ifosfamide and mesna were administered; carboplatin was administered to achieve an AUC of 4 mg x min/mL. Plasma samples were collected and assayed by reverse-phase high-performance liquid chromatography (HPLC) for total mesna and total cysteine concentrations at 0, 1, 3, 6, 24, 25, 28, and 48 hours. A one-compartment pharmacokinetic model was fit to the mesna plasma concentrations, using M.A.P. Bayesian estimation (ADAPT II). Pharmacodynamics were evaluated by fitting an inhibitory Emax model to the cysteine concentration data. Both the pharmacokinetic (median R2 = 0.95; range = 0.85-0.98) and pharmacodynamic (median R2 = 0.96; range = 0.74-1.0) models fit the data well. Mean (coefficient of variation [CV%]) mesna pharmacokinetic parameter estimates were as follows: Vss of 15.3 (29) L/m2, CL of 4.6 (29) L/h/m2, and half-life of 2.2 (37) hours. Mean (CV%) pharmacodynamic parameter estimates were as follows: Emax of 31.7 (19) microg/mL and EC50 of 10.3 (52) microg/mL. Mesna produced a rapid, concentration-dependent reduction in plasma cysteine concentrations that could be adequately characterized by an inhibitory Emax pharmacodynamic model. The depletion of plasma cysteine was facilitated by ifosfamide, suggesting a pharmacodynamic interaction between these two agents. Further increases in mesna doses beyond those administered in this study would be unlikely to provide additional benefit.
Talbot SM, Rankin C, Taub RN, et al.
High-dose ifosfamide with mesna and granulocyte-colony-stimulating factor (recombinant human G-CSF) in patients with unresectable malignant mesothelioma.
Cancer. 2003; 98(2):331-6 [PubMed] Related Publications
High-dose ifosfamide with mesna and granulocyte-colony-stimulating factor (recombinant human G-CSF) in patients with unresectable malignant mesothelioma.
Cancer. 2003; 98(2):331-6 [PubMed] Related Publications
BACKGROUND: The current study was conducted to assess the activity and toxicity of high-dose ifosfamide and mesna with recombinant human granulocyte-colony-stimulating factor (rhG-CSF), given in an outpatient setting, in the treatment of patients with unresectable malignant mesothelioma.
METHODS: Between September 1994 and September 1996, 41 patients with histologically verified, unresectable malignant mesothelioma were registered, 38 of whom were analyzable (2 were ineligible and 1 was nonanalyzable). Patients received intravenous ifosfamide at a dose of 2.8 g/m2 over 3 hours (total dose of 14 g/m2), plus mesna at a dose of 0.56 g/m2 prior to and at 4 hours and 8 hours after ifosfamide infusion daily for 5 days every 21 days. rhG-CSF at a dose of 5 microg/kg/day was administered subcutaneously on days 6-15.
RESULTS: Response assessment could be determined adequately in 21 patients. Two patients obtained responses; 1 was a confirmed partial response (3%; 95% confidence interval [95% CI], 0-14%) and 1 was an unconfirmed response (3%; 95% CI, 5-14%). Eleven patients had stable disease (29%), 7 patients developed disease progression (18%), 1 patient had an early death (3%), and 17 patients had inadequate assessment (45%). At the time of last follow-up, 36 of the 38 eligible patients had developed disease progression, with a median progression-free survival of 5 months (95% CI, 3-7 months) and 34 patients had died with a median survival of 7 months (95% CI, 6-9 months). Twenty-four patients (63%) and 7 patients (18%), respectively, had Grade (according to Southwestern Oncology Group Toxicity Criteria) 4 hematologic toxicities and Grade 4 nonhematological toxicities. There was one treatment-related death, the result of infection, pulmonary edema, and renal failure.
CONCLUSIONS: This regimen demonstrated a low overall objective response rate with substantial toxicity, and in the opinion of the authors does not warrant further investigation in the treatment of patients with unresectable malignant mesothelioma.
METHODS: Between September 1994 and September 1996, 41 patients with histologically verified, unresectable malignant mesothelioma were registered, 38 of whom were analyzable (2 were ineligible and 1 was nonanalyzable). Patients received intravenous ifosfamide at a dose of 2.8 g/m2 over 3 hours (total dose of 14 g/m2), plus mesna at a dose of 0.56 g/m2 prior to and at 4 hours and 8 hours after ifosfamide infusion daily for 5 days every 21 days. rhG-CSF at a dose of 5 microg/kg/day was administered subcutaneously on days 6-15.
RESULTS: Response assessment could be determined adequately in 21 patients. Two patients obtained responses; 1 was a confirmed partial response (3%; 95% confidence interval [95% CI], 0-14%) and 1 was an unconfirmed response (3%; 95% CI, 5-14%). Eleven patients had stable disease (29%), 7 patients developed disease progression (18%), 1 patient had an early death (3%), and 17 patients had inadequate assessment (45%). At the time of last follow-up, 36 of the 38 eligible patients had developed disease progression, with a median progression-free survival of 5 months (95% CI, 3-7 months) and 34 patients had died with a median survival of 7 months (95% CI, 6-9 months). Twenty-four patients (63%) and 7 patients (18%), respectively, had Grade (according to Southwestern Oncology Group Toxicity Criteria) 4 hematologic toxicities and Grade 4 nonhematological toxicities. There was one treatment-related death, the result of infection, pulmonary edema, and renal failure.
CONCLUSIONS: This regimen demonstrated a low overall objective response rate with substantial toxicity, and in the opinion of the authors does not warrant further investigation in the treatment of patients with unresectable malignant mesothelioma.
Benassi L, Benassi G, Kaihura CT, et al.
Chemically assisted dissection of tissues in laparoscopic excision of endometriotic cysts.
J Am Assoc Gynecol Laparosc. 2003; 10(2):205-9 [PubMed] Related Publications
Chemically assisted dissection of tissues in laparoscopic excision of endometriotic cysts.
J Am Assoc Gynecol Laparosc. 2003; 10(2):205-9 [PubMed] Related Publications
STUDY OBJECTIVE: To evaluate the capacity of chemical dissection of tissues using a mucolytic substance, Mesna, in improving laparoscopic excision of endometriotic cysts.
DESIGN: Randomized, double-blind, controlled trial (Canadian Task Force classification I).
SETTING: University-affiliated training hospital.
PATIENTS: Forty-four women with symptomatic ovarian endometriotic cysts. Intervention. Laparoscopic excision of endometriotic cysts in 22 women with the aid of Mesna solution and in 22 with the aid of saline solution.
MEASUREMENTS AND MAIN RESULTS: In comparison with saline solution, Mesna as a chemical dissector resulted in significant reductions in operating time, in difficulty encountered by the surgeon to enucleate the cysts, and in less bleeding. No differences were found in length of hospital stay, costs of surgeries, analgesic requirement, and fever. Postoperatively, patients treated with Mesna achieved more pregnancies than those treated with saline.
CONCLUSION: Chemical dissection of tissues with Mesna proved to be a safe and suitable support in laparoscopic surgery for ovarian endometriotic cysts.
DESIGN: Randomized, double-blind, controlled trial (Canadian Task Force classification I).
SETTING: University-affiliated training hospital.
PATIENTS: Forty-four women with symptomatic ovarian endometriotic cysts. Intervention. Laparoscopic excision of endometriotic cysts in 22 women with the aid of Mesna solution and in 22 with the aid of saline solution.
MEASUREMENTS AND MAIN RESULTS: In comparison with saline solution, Mesna as a chemical dissector resulted in significant reductions in operating time, in difficulty encountered by the surgeon to enucleate the cysts, and in less bleeding. No differences were found in length of hospital stay, costs of surgeries, analgesic requirement, and fever. Postoperatively, patients treated with Mesna achieved more pregnancies than those treated with saline.
CONCLUSION: Chemical dissection of tissues with Mesna proved to be a safe and suitable support in laparoscopic surgery for ovarian endometriotic cysts.
Pendyala L, Schwartz G, Smith P, et al.
Modulation of plasma thiols and mixed disulfides by BNP7787 in patients receiving paclitaxel/cisplatin therapy.
Cancer Chemother Pharmacol. 2003; 51(5):376-84 [PubMed] Related Publications
Modulation of plasma thiols and mixed disulfides by BNP7787 in patients receiving paclitaxel/cisplatin therapy.
Cancer Chemother Pharmacol. 2003; 51(5):376-84 [PubMed] Related Publications
BACKGROUND: BNP7787 (disodium 2,2'-dithio-bis-ethane sulfonate) was evaluated in a phase I clinical trial with paclitaxel and cisplatin to assess the safety and potential efficacy for preventing or reducing cisplatin- and paclitaxel-induced toxicities. During this trial the effects of BNP7787 administration on the total concentrations (oxidized plus free) of cysteine, homocysteine and GSH in plasma, free and total GSH in WBC and rate of urinary excretion of cysteine were studied. The pharmacokinetics of ultrafilterable (free, non-protein bound) platinum were also determined after cisplatin (75 mg/m(2)) treatment which followed paclitaxel (175 mg/m(2)) and BNP7787 (8.2 to 27.6 g/m(2)).
METHODS: Plasma thiols were measured by HPLC with fluorescence detection and platinum was measured by atomic absorption spectrophotometry.
RESULTS: BNP7787 administration produced a significant depletion of all plasma thiols in all the patients studied. Differences were noted in the kinetics of BNP7787-induced depletion of cysteine and other thiols. A significant depletion of cysteine occurred with a time lag of about 2 h after the end of BNP7787 infusion, while a reversible depletion of GSH and homocysteine occurred immediately following the start of BNP7787 infusion, with the plasma thiol/disulfide nadir corresponding to the end of infusion. The mean half-life of cysteine depletion following BNP7787 administration was 2.2 h, significantly longer than for homocysteine (0.23 h), or GSH (0.18 h; P<0.05 for both). A several-fold increase in the urinary excretion of cysteine occurred following BNP7787 administration in all patients. The BNP7787-induced thiol/disulfide depletion in plasma was not affected by cisplatin administration ( P>0.05). BNP7787 administration had no effect on the ultrafilterable platinum pharmacokinetics. The 2-h lag in the depletion of cysteine, the most abundant thiol in plasma, suggests that the process may be related to the formation of free mesna from BNP7787 and that increased levels of mesna are not in circulation until after 2 h after BNP7787 administration. No effect of BNP7787 was seen on the GSH concentration in WBC, possibly reflecting the inability of these cells to take up BNP7787.
CONCLUSION: The results suggest that BNP7787 has the potential to enhance cisplatin antitumor activity by depleting the reactive thiols in plasma.
METHODS: Plasma thiols were measured by HPLC with fluorescence detection and platinum was measured by atomic absorption spectrophotometry.
RESULTS: BNP7787 administration produced a significant depletion of all plasma thiols in all the patients studied. Differences were noted in the kinetics of BNP7787-induced depletion of cysteine and other thiols. A significant depletion of cysteine occurred with a time lag of about 2 h after the end of BNP7787 infusion, while a reversible depletion of GSH and homocysteine occurred immediately following the start of BNP7787 infusion, with the plasma thiol/disulfide nadir corresponding to the end of infusion. The mean half-life of cysteine depletion following BNP7787 administration was 2.2 h, significantly longer than for homocysteine (0.23 h), or GSH (0.18 h; P<0.05 for both). A several-fold increase in the urinary excretion of cysteine occurred following BNP7787 administration in all patients. The BNP7787-induced thiol/disulfide depletion in plasma was not affected by cisplatin administration ( P>0.05). BNP7787 administration had no effect on the ultrafilterable platinum pharmacokinetics. The 2-h lag in the depletion of cysteine, the most abundant thiol in plasma, suggests that the process may be related to the formation of free mesna from BNP7787 and that increased levels of mesna are not in circulation until after 2 h after BNP7787 administration. No effect of BNP7787 was seen on the GSH concentration in WBC, possibly reflecting the inability of these cells to take up BNP7787.
CONCLUSION: The results suggest that BNP7787 has the potential to enhance cisplatin antitumor activity by depleting the reactive thiols in plasma.
Emmanouilides C, Lill M, Telatar M, et al.
Mitoxantrone/ifosfamide/etoposide salvage regimen with rituximab for in vivo purging in patients with relapsed lymphoma.
Clin Lymphoma. 2002; 3(2):111-6 [PubMed] Related Publications
Mitoxantrone/ifosfamide/etoposide salvage regimen with rituximab for in vivo purging in patients with relapsed lymphoma.
Clin Lymphoma. 2002; 3(2):111-6 [PubMed] Related Publications
Treatment with the anti-CD20 antibody rituximab prior to stem cell collection may lead to tumor-free stem cell collections in patients with B-cell lymphoma undergoing autologous stem cell transplantation. To test the feasibility of obtaining polymerase chain reaction (PCR)-negative stem cell collection, 30 patients with a variety of B-cell lymphomas were enrolled in a protocol employing a common MINE (mitoxantrone/ifosfamide/etoposide) salvage regimen with rituximab (in vivo purging). Rituximab 400 mg/m2 was administered weekly for 3 weeks on days 1, 6, and 8 in relation to the last MINE cycle, which was followed by growth factor-stimulated peripheral stem cell collection. The median number of CD34(+) cells/kg was 2.5 million cells/kg collected over a median of 5 days. Polymerase chain reaction amplification for the t (14;18) or the heavy-chain gene rearrangement was performed prior to treatment and on the leukapheresis sample. Out of 15 patients who had a positive PCR signal prior to treatment, 10 had PCR-negative stem cell collections, whereas 5 had PCR-positive stem cell collections. After high-dose chemotherapy and stem cell transplant, all patients with a PCR-positive signal pretreatment became PCR negative. We conclude that rituximab may increase the yield of tumor-free stem cells. Higher rates of PCR negativity have been reported when more intense and protracted chemoimmunotherapy regimens have been employed. The magnitude of clinical benefit and the significance of the PCR analysis in stem cells after rituximab requires larger studies.
Cohen MH, Dagher R, Griebel DJ, et al.
U.S. Food and Drug Administration drug approval summaries: imatinib mesylate, mesna tablets, and zoledronic acid.
Oncologist. 2002; 7(5):393-400 [PubMed] Related Publications
U.S. Food and Drug Administration drug approval summaries: imatinib mesylate, mesna tablets, and zoledronic acid.
Oncologist. 2002; 7(5):393-400 [PubMed] Related Publications
The purpose of this report is to summarize information on drugs recently approved by the U.S. Food and Drug Administration. Three drugs have recently been approved: Gleevec (imatinib mesylate) at a starting dose of 400 or 600 mg daily for the treatment of malignant unresectable and/or metastatic gastrointestinal stromal tumors; Mesnex (mesna) tablets as a prophylactic agent to reduce the incidence of ifosfamide-induced hemorrhagic cystitis, and Zometa (zoledronic acid) for the treatment of patients with multiple myeloma and for patients with documented bone metastases from solid tumors, in conjunction with standard antineoplastic therapy. Prostate cancer should have progressed after treatment with at least one hormonal therapy. The recommended dose and schedule is 4 mg infused over 15 minutes every 3-4 weeks. These three drugs represent three different types of drug approval: Gleevec is an accelerated approval and supplemental new drug application (NDA); Mesnex tablets represent an oral formulation of a drug approved 14 years ago as an intravenous formulation, and Zometa represents a standard NDA for a noncytotoxic, supportive-care drug. Information provided includes rationale for drug development, study design, efficacy and safety results, and pertinent literature references.
Hawkins DS, Felgenhauer J, Park J, et al.
Peripheral blood stem cell support reduces the toxicity of intensive chemotherapy for children and adolescents with metastatic sarcomas.
Cancer. 2002; 95(6):1354-65 [PubMed] Related Publications
Peripheral blood stem cell support reduces the toxicity of intensive chemotherapy for children and adolescents with metastatic sarcomas.
Cancer. 2002; 95(6):1354-65 [PubMed] Related Publications
BACKGROUND: To increase the dose intensity (DI) of chemotherapy for pediatric patients with metastatic sarcomas, including the Ewing sarcoma family of tumors (ESFT) and rhabdomyosarcoma (RMS), the authors tested the feasibility of an intensive regimen supported by granulocyte-colony stimulating factor (G-CSF) and peripheral blood stem cells (PBSC).
METHODS: Twenty-three children and adolescents with metastatic sarcomas received vincristine, doxorubicin, cyclophosphamide, ifosfamide, sodium mercaptoethanesulfonate (mensa), and etoposide (VACIME) chemotherapy, consisting of 8 courses of vincristine 2 mg/m(2) on Day 0, doxorubicin 37.5 mg/m(2) per day on Days 0-1, cyclophosphamide 360 mg/m(2) per day on Days 0-4, ifosfamide 1800 mg/m(2) per day on Days 0-4, mesna 2400 mg/m(2) per day, and etoposide 100 mg/m(2) per day on Days 0-4. Doxorubicin was omitted in Courses 7 and 8. G-CSF was given after each course of therapy. Courses of therapy were repeated every 21 days or as soon as hematopoietic recovery permitted. PBSC were collected twice: first, after Course 2 (infused after Courses 3 and 4) and, second, after Course 4 (infused after Courses 5 and 6). Surgical resection followed Course 6, and radiotherapy followed Course 8.
RESULTS: PBSC collections were adequate in 91% of all harvests. The mean DI was 82% (standard deviation, 14%) of the intended DI, which was greater than historic data without PBSC support. Seventeen patients (74%) achieved a complete response (CR), 12 patients with chemotherapy alone and 5 more patients after undergoing surgical resection. Fifteen patients developed progressive disease, with a 2-year event free survival (EFS) rate of 39% (95% confidence interval, 19-59%). Hematopoietic toxicity was severe and cumulative, although it was less than that seen previously without PBSC support.
CONCLUSIONS: PBSC-supported multicycle chemotherapy is a feasible method to increase chemotherapy DI for pediatric patients with metastatic sarcomas. Although the CR rate compared favorably with previously reported response rates, the 2-year EFS rate was similar to that achieved with other intensive regimens.
METHODS: Twenty-three children and adolescents with metastatic sarcomas received vincristine, doxorubicin, cyclophosphamide, ifosfamide, sodium mercaptoethanesulfonate (mensa), and etoposide (VACIME) chemotherapy, consisting of 8 courses of vincristine 2 mg/m(2) on Day 0, doxorubicin 37.5 mg/m(2) per day on Days 0-1, cyclophosphamide 360 mg/m(2) per day on Days 0-4, ifosfamide 1800 mg/m(2) per day on Days 0-4, mesna 2400 mg/m(2) per day, and etoposide 100 mg/m(2) per day on Days 0-4. Doxorubicin was omitted in Courses 7 and 8. G-CSF was given after each course of therapy. Courses of therapy were repeated every 21 days or as soon as hematopoietic recovery permitted. PBSC were collected twice: first, after Course 2 (infused after Courses 3 and 4) and, second, after Course 4 (infused after Courses 5 and 6). Surgical resection followed Course 6, and radiotherapy followed Course 8.
RESULTS: PBSC collections were adequate in 91% of all harvests. The mean DI was 82% (standard deviation, 14%) of the intended DI, which was greater than historic data without PBSC support. Seventeen patients (74%) achieved a complete response (CR), 12 patients with chemotherapy alone and 5 more patients after undergoing surgical resection. Fifteen patients developed progressive disease, with a 2-year event free survival (EFS) rate of 39% (95% confidence interval, 19-59%). Hematopoietic toxicity was severe and cumulative, although it was less than that seen previously without PBSC support.
CONCLUSIONS: PBSC-supported multicycle chemotherapy is a feasible method to increase chemotherapy DI for pediatric patients with metastatic sarcomas. Although the CR rate compared favorably with previously reported response rates, the 2-year EFS rate was similar to that achieved with other intensive regimens.
Boven E, Verschraagen M, Hulscher TM, et al.
BNP7787, a novel protector against platinum-related toxicities, does not affect the efficacy of cisplatin or carboplatin in human tumour xenografts.
Eur J Cancer. 2002; 38(8):1148-56 [PubMed] Related Publications
BNP7787, a novel protector against platinum-related toxicities, does not affect the efficacy of cisplatin or carboplatin in human tumour xenografts.
Eur J Cancer. 2002; 38(8):1148-56 [PubMed] Related Publications
BNP7787 (2',2'-dithio-bis-ethane sulphonate sodium), a water-soluble disulphide, is chemically and mechanistically different from other sulphur-containing chemoprotective agents. Presently, BNP7787 is under investigation for its protective properties with regard to the side-effects of platinum compounds. In this study, we evaluated BNP7787, mesna and amifostine for their effects on the antitumour activity of platinum compounds. Continuous exposure to BNP7787 did not affect the antiproliferative effects of cisplatin or carboplatin, but the efficacy of both compounds was reduced in the presence of mesna in vitro in two human ovarian cancer cell lines. BNP7787 or amifostine combined with cisplatin or carboplatin given in standard schedules for the treatment of nude mice bearing well-established OVCAR-3 xenografts did not interfere with platinum-induced inhibition of tumour growth. Of interest, BNP7787 or amifostine co-administered with carboplatin was significantly more effective than carboplatin alone (P<0.01). In the presence of amifostine, doses of cisplatin and carboplatin could be safely increased by factors of 1.6 and 1.5, respectively. Unlike in a previous study of BNP7787 in tumour-bearing rats, BNP7787 did not protect against additional weight loss following treatment with higher doses of cisplatin in OVCAR-3-bearing mice. Pharmacokinetics of (mixed) disulphides including BNP7787 and extractable mesna in deproteinised plasma revealed a rapid disappearance of BNP7787 and an AUC(5-60) value of mesna 9-fold lower than that calculated after an equivalent dose of mesna by weight. We can conclude that BNP7787 does not interfere with the antitumour activity of platinum compounds in vitro and in vivo. Clinical trials are underway to evaluate the protection of normal tissues by BNP7787 when combined with cisplatin.
Souid AK, Fahey RC, Aktas MK, et al.
Blood thiols following amifostine and mesna infusions, a pediatric oncology group study.
Drug Metab Dispos. 2001; 29(11):1460-6 [PubMed] Related Publications
Blood thiols following amifostine and mesna infusions, a pediatric oncology group study.
Drug Metab Dispos. 2001; 29(11):1460-6 [PubMed] Related Publications
The Pediatric Oncology Group study for metastatic Ewing's sarcoma used amifostine and mesna with the alkylating agents. To determine the fate of combined drug thiols, we measured thiol levels in plasma, red blood cells (RBC), and peripheral blood mononuclear cells (PBMC) of four patients. We also conducted analogous measurements on two patients who received mesna alone and a volunteer's blood following in vitro treatment. Thiols were labeled with monobromobimane, separated on high-pressure liquid chromatography, and detected by fluorescence. Incubation of a volunteer's blood with mesna, WR-1065, or both revealed that cellular uptake of total reducible drug was approximately 10% of plasma level for mesna but approximately 60% for WR-1065. Cellular drugs were mainly the thiol form, whereas half of the plasma drugs were disulfides. Combined incubation with both thiols did not change the extent or form of uptake. WR-1065 and mesna prevented glutathione depletion by 4-hydroperoxycyclophosphamide. Results from patients were similar. WR-1065 and mesna appeared in the cells by the end of the drug infusions, although WR-1065 uptake was more efficient than mesna. The concentration-time profiles of mesna in RBC paralleled those in plasma. Amifostine administration during mesna infusion caused transient increase in mesna levels. Both agents increased blood cysteine and decreased total reducible cysteine. Mesna alone and mesna plus amifostine prevented cellular glutathione depletion. In conclusion, mesna is imported by RBC and PBMC, but less efficiently than WR-1065. When present at equal levels, these thiols do not influence each other's uptake. Adequate dosing of either drug is necessary for protecting the cells from toxic effects of alkylating agents.



 Dacarbazine
Dacarbazine