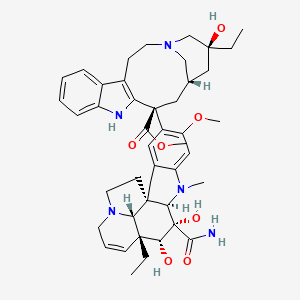
Vindesine
"Vinblastine derivative with antineoplastic activity against CANCER. Major side effects are myelosuppression and neurotoxicity. Vindesine is used extensively in chemotherapy protocols (ANTINEOPLASTIC COMBINED CHEMOTHERAPY PROTOCOLS)." (MeSH 2013)
Found this page useful?
 Web Resources: Vindesine
Web Resources: Vindesine Latest Research Publications
Latest Research PublicationsWeb Resources: Vindesine (5 links)
Cancer Research UK
Macmillan Cancer Support
NHS Evidence
PubChem
Irish Cancer Society
Latest Research Publications
This list of publications is regularly updated (Source: PubMed).
Lei X, Chen M, Huang M, et al.
Desacetylvinblastine Monohydrazide Disrupts Tumor Vessels by Promoting VE-cadherin Internalization.
Theranostics. 2018; 8(2):384-398 [PubMed] Free Access to Full Article Related Publications
Desacetylvinblastine Monohydrazide Disrupts Tumor Vessels by Promoting VE-cadherin Internalization.
Theranostics. 2018; 8(2):384-398 [PubMed] Free Access to Full Article Related Publications
Vinca alkaloids, the well-known tubulin-binding agents, are widely used for the clinical treatment of malignant tumors. However, little attention has been paid to their vascular disrupting effects, and the underlying mechanisms remain largely unknown. This study aims to investigate the vascular disrupting effect and the underlying mechanisms of vinca alkaloids.
Zhou H, Li L, Zhou Y, Han Y
Syndrome of inappropriate antidiuretic hormone secretion from concomitant use of itraconazole and vindesine.
J Clin Pharm Ther. 2018; 43(1):137-140 [PubMed] Related Publications
Syndrome of inappropriate antidiuretic hormone secretion from concomitant use of itraconazole and vindesine.
J Clin Pharm Ther. 2018; 43(1):137-140 [PubMed] Related Publications
WHAT IS KNOWN AND OBJECTIVE: Several studies have reported that itraconazole-induced inhibition of vincristine (VCR) metabolism might result in neurological impairment and syndrome of inappropriate antidiuretic hormone (SIADH). However, there are few reports concerning adverse drug reactions (ADRs) resulting from concomitant use of vindesine (VDS) and itraconazole. Here, we report the first case of adverse drug interactions (ADIs) between itraconazole and VDS in a Chinese child with acute lymphocytic leukaemia (ALL).
CASE SUMMARY: A 4-year-old boy was diagnosed with standard-risk ALL and was receiving VDS (3 mg/m
WHAT IS NEW AND CONCLUSION: Syndrome of inappropriate antidiuretic hormone from co-administration of itraconazole and VDS has not previously been reported to our knowledge. We suggest that the concomitant use of these drugs should be avoided if possible. The use of alternative antifungal drugs (AFDs) should be considered, and ADRs should be closely monitored when the combination of itraconazole and VDS is unavoidable.
CASE SUMMARY: A 4-year-old boy was diagnosed with standard-risk ALL and was receiving VDS (3 mg/m
WHAT IS NEW AND CONCLUSION: Syndrome of inappropriate antidiuretic hormone from co-administration of itraconazole and VDS has not previously been reported to our knowledge. We suggest that the concomitant use of these drugs should be avoided if possible. The use of alternative antifungal drugs (AFDs) should be considered, and ADRs should be closely monitored when the combination of itraconazole and VDS is unavoidable.
Zhu RH, Li HD, Cai HL, et al.
Validated HILIC-MS/MS assay for determination of vindesine in human plasma: Application to a population pharmacokinetic study.
J Pharm Biomed Anal. 2014; 96:31-6 [PubMed] Related Publications
Validated HILIC-MS/MS assay for determination of vindesine in human plasma: Application to a population pharmacokinetic study.
J Pharm Biomed Anal. 2014; 96:31-6 [PubMed] Related Publications
The first HILIC-tandem mass spectrometry (MS/MS) method for determination of vindesine (VDS) in human plasma using vinorelbine as an internal standard (IS) has been developed and validated. Plasma samples clean-up consisted of solid phase extraction with a strata™-X column. The compounds were separated on a HILIC column with an isocratic mobile phase consisting of acetonitrile and 15mM ammonium acetate buffer containing 0.15% formic acid (80:20, v/v). The detection was performed on a triple quadrupole tandem mass spectrometer via electrospray positive ionization (ESI(+)). The ion transitions recorded in multiple reaction monitoring mode were m/z 754.6→123.8 for VDS and 779.4→323.3 for IS, respectively. Linear calibration curves were obtained in the concentration range of 0.3-28ng/ml and the lower limit of quantification for VDS was 0.3ng/ml. The coefficient of variation of the assay precision was less than 13%, and the accuracy exceeded 96%. The developed assay method was successfully applied for the evaluation of population pharmacokinetics of VDS after intravenous infusion of Xi Ai Ke Vial(®) (3mg of Vindesine Sulfate for Injection) to Chinese Han subjects with hematological malignant disorders.
Ichikawa M, Suzuki D, Inamoto J, et al.
Successful alternative treatment containing vindesine for acute lymphoblastic leukemia with Charcot-Marie-Tooth disease.
J Pediatr Hematol Oncol. 2012; 34(3):239-41 [PubMed] Related Publications
Successful alternative treatment containing vindesine for acute lymphoblastic leukemia with Charcot-Marie-Tooth disease.
J Pediatr Hematol Oncol. 2012; 34(3):239-41 [PubMed] Related Publications
Peripheral neuropathy is a well-known side effect of vincristine (VCR), a microtubule inhibitor commonly used to treat malignancies. Severe neurological adverse events can occur in patients with Charcot-Marie-Tooth disease (CMT) treated with VCR. Vindesine is also a microtubule inhibitor, which, like VCR, is widely used to treat malignancies. The case of an 11-year-old female patient with CMT type 1A who developed severe peripheral neuropathy induced by VCR given for her acute lymphoblastic leukemia is reported. Alternative treatment containing vindesine instead of VCR led to a successful outcome without a relapse of leukemia or neurological worsening of CMT.
Liu L, Shi B, Ye L, et al.
Vindesine-induced neuropathy mimicking Guillain-Barré syndrome.
Leuk Res. 2009; 33(12):e232-3 [PubMed] Related Publications
Vindesine-induced neuropathy mimicking Guillain-Barré syndrome.
Leuk Res. 2009; 33(12):e232-3 [PubMed] Related Publications
A 13-year-old girl with acute lymphoblastic leukaemia, who was being treated with chemotherapy (including vindesine), developed paraplegia without paresthesia, which mimic Guillain-Barré syndrome. Spinal fluid analysis showed a normal protein level, vindesine neuropathy seemed to be the cause of the patient's neurological symptoms. The patient seemed to benefit from human normal immunoglobulin therapy and recovered 4 weeks later.
Rhomberg W, Wink A, Pokrajac B, et al.
Treatment of vascular soft tissue sarcomas with razoxane, vindesine, and radiation.
Int J Radiat Oncol Biol Phys. 2009; 74(1):187-91 [PubMed] Related Publications
Treatment of vascular soft tissue sarcomas with razoxane, vindesine, and radiation.
Int J Radiat Oncol Biol Phys. 2009; 74(1):187-91 [PubMed] Related Publications
PURPOSE: In previous studies, razoxane and vindesine together with radiotherapy was proved to be effective in soft tissue sarcomas (STS). Because razoxane leads to a redifferentiation of pathological tumor blood vessels, it was of particular interest to study the influence of this drug combination in vascular soft tissue sarcomas.
METHODS AND MATERIALS: This open multicenter Phase II study was performed by the Austrian Society of Radiooncology. Among 13 evaluable patients (10 angiosarcomas and 3 hemangio-pericytomas), 9 had unresectable measurable disease, 3 showed microscopic residuals, and 1 had a resection with clear margins. They received a basic treatment with razoxane and vindesine supported by radiation therapy. Outcome measures were objective response rates, survival time, and the incidence of distant metastases.
RESULTS: In nine patients with measurable vascular soft tissue sarcomas (eight angiosarcomas and one hemangiopericytoma), 6 complete remissions, 2 partial remissions, and 1 minor remission were achieved, corresponding to a major response rate of 89%. A maintenance therapy with razoxane and vindesine of 1 year or longer led to a suppression of distant metastases. The median survival time from the start of the treatment is 23+ months (range, 3-120+) for 12 patients with macroscopic and microscopic residual disease. The progression-free survival at 6 months was 75%. The combined treatment was associated with a low general toxicity, but attention must be given to increased normal tissue reactions.
CONCLUSIONS: This trimodal treatment leads to excellent response rates, and it suppresses distant metastases when given as maintenance therapy.
METHODS AND MATERIALS: This open multicenter Phase II study was performed by the Austrian Society of Radiooncology. Among 13 evaluable patients (10 angiosarcomas and 3 hemangio-pericytomas), 9 had unresectable measurable disease, 3 showed microscopic residuals, and 1 had a resection with clear margins. They received a basic treatment with razoxane and vindesine supported by radiation therapy. Outcome measures were objective response rates, survival time, and the incidence of distant metastases.
RESULTS: In nine patients with measurable vascular soft tissue sarcomas (eight angiosarcomas and one hemangiopericytoma), 6 complete remissions, 2 partial remissions, and 1 minor remission were achieved, corresponding to a major response rate of 89%. A maintenance therapy with razoxane and vindesine of 1 year or longer led to a suppression of distant metastases. The median survival time from the start of the treatment is 23+ months (range, 3-120+) for 12 patients with macroscopic and microscopic residual disease. The progression-free survival at 6 months was 75%. The combined treatment was associated with a low general toxicity, but attention must be given to increased normal tissue reactions.
CONCLUSIONS: This trimodal treatment leads to excellent response rates, and it suppresses distant metastases when given as maintenance therapy.
Eigentler TK, Radny P, Hauschild A, et al.
Adjuvant treatment with vindesine in comparison to observation alone in patients with metastasized melanoma after complete metastasectomy: a randomized multicenter trial of the German Dermatologic Cooperative Oncology Group.
Melanoma Res. 2008; 18(5):353-8 [PubMed] Related Publications
Adjuvant treatment with vindesine in comparison to observation alone in patients with metastasized melanoma after complete metastasectomy: a randomized multicenter trial of the German Dermatologic Cooperative Oncology Group.
Melanoma Res. 2008; 18(5):353-8 [PubMed] Related Publications
To evaluate the efficacy and safety of vindesine in patients with metastatic melanoma after complete metastasectomy. One hundred and forty-two patients with metastatic spread to regional sites, lymph nodes, and distant sites after complete metastasectomy were randomized to receive either treatment with vindesine for 2 years or observation alone. Vindesine 3 mg/m intravenously was administered biweekly for the first 26 weeks following 3-week intervals for an additional 26 weeks and thereafter every 4 weeks for 52 weeks. One hundred and thirty-nine patients were eligible for intent-to-treat analysis. Median follow-up time was 46 months. Median recurrence free survival was 7.9 months in the vindesine group and 7.6 months in the observational group (P=0.40). Three-year overall survival rate was 54.9% (37 patients) for patients receiving vindesine in comparison to 43.6% (31 patients) in the observation arm (P=0.07). No grade IV toxicity was observed. The two major side effects in the vindesine group were alopecia and peripheral neuropathy. Ten patients went off treatment because of grade III toxicity. Adjuvant treatment with vindesine did not significantly prolong disease free or overall survival in high-risk melanoma patients. Thus, this randomized trial did not confirm earlier reports of beneficial effects of adjuvant vindesine and can therefore not be recommended.
Rhomberg W, Eiter H, Schmid F, Saely C
Razoxane and vindesine in advanced soft tissue sarcomas: impact on metastasis, survival and radiation response.
Anticancer Res. 2007 Sep-Oct; 27(5B):3609-14 [PubMed] Related Publications
Razoxane and vindesine in advanced soft tissue sarcomas: impact on metastasis, survival and radiation response.
Anticancer Res. 2007 Sep-Oct; 27(5B):3609-14 [PubMed] Related Publications
BACKGROUND: The treatment options in advanced soft tissue sarcomas (STS) are limited. In a pilot study, an antimetastatic and radiosensitizing treatment concept was explored.
PATIENTS AND METHODS: Twenty-one patients with unresectable and/or oligometastatic STS received the drugs razoxane and vindesine supported by radiotherapy and surgery. Long-term treatment was intended in metastatic disease. Forty-one patients with comparable stages of STS treated with contemporary chemotherapy served as non-randomised controls. The prognostic parameters of the groups were comparable.
RESULTS: In the study group, the median number of new metastases after 6 months was 0 (range, 0-40) and after 9 months likewise 0 (0-70). The corresponding numbers in the control group were 4.5 (range, 0-40) and 9 (0->100) (p<0.001). The progression-free survival at 6 months was 71% in the study group and 23% in the controls, and the median survival time from the occurrence of the first metastasis was 16 months versus 9 months. The rate of major responses under radiotherapy combined with razoxane and vindesine was 88%, and in the control group 62% (p=0.007). The combined treatment was associated with a low to moderate toxicity.
CONCLUSION: The treatment combination inhibited the development of remote metastases in the majority of patients with STS and prolonged survival to some extent.
PATIENTS AND METHODS: Twenty-one patients with unresectable and/or oligometastatic STS received the drugs razoxane and vindesine supported by radiotherapy and surgery. Long-term treatment was intended in metastatic disease. Forty-one patients with comparable stages of STS treated with contemporary chemotherapy served as non-randomised controls. The prognostic parameters of the groups were comparable.
RESULTS: In the study group, the median number of new metastases after 6 months was 0 (range, 0-40) and after 9 months likewise 0 (0-70). The corresponding numbers in the control group were 4.5 (range, 0-40) and 9 (0->100) (p<0.001). The progression-free survival at 6 months was 71% in the study group and 23% in the controls, and the median survival time from the occurrence of the first metastasis was 16 months versus 9 months. The rate of major responses under radiotherapy combined with razoxane and vindesine was 88%, and in the control group 62% (p=0.007). The combined treatment was associated with a low to moderate toxicity.
CONCLUSION: The treatment combination inhibited the development of remote metastases in the majority of patients with STS and prolonged survival to some extent.
Yoshida M, Matsui Y, Ikarashi Y, et al.
Antiproliferating activity of the mitotic inhibitor pironetin against vindesine- and paclitaxel-resistant human small cell lung cancer H69 cells.
Anticancer Res. 2007 Mar-Apr; 27(2):729-36 [PubMed] Related Publications
Antiproliferating activity of the mitotic inhibitor pironetin against vindesine- and paclitaxel-resistant human small cell lung cancer H69 cells.
Anticancer Res. 2007 Mar-Apr; 27(2):729-36 [PubMed] Related Publications
Pironetin, isolated from Streptomyces sp., is a potent inhibitor of microtubule assembly and the first compound identified that covalently binds to alpha-tubulin at Lys352. We examined whether pironetin is an effective agent against human small cell lung cancer H69 cells, including two cell lines resistant to the microtubule-targeted drugs vindesine (H69/VDS) and paclitaxel (H69/Txl) that interact with beta-tubulin. Pironetin was found to be effective against these resistant cells as well as their parental cells. In addition, pironetin inhibited the growth of human leukemic K562 multidrug-resistant cells (K562/ADM), which have mdr1 gene expression, as well as the parental K562 cells. In these cell lines, including the parental and resistant cells, pironetin caused complete mitotic arrest; in addition, apoptosis inductions by 30 and 100 nM pironetin were observed. In this study, the new mitotic inhibitor, pironetin, was found to be effective not only against human tumor cell lines resistant to microtubule-targeted drugs, but also multidrug-resistant cells with mdr1 gene expression. These results suggest that pironetin is a useful agent for overcoming drug resistance in cancer chemotherapy.
Santo A, Genestreti G, Terzi A, et al.
Gemcitabine (GEM) and vindesine (VDS) in advanced non-small cell lung cancer (NSCLC): a phase II study in elderly or poor performance status patients.
Lung Cancer. 2006; 53(3):355-60 [PubMed] Related Publications
Gemcitabine (GEM) and vindesine (VDS) in advanced non-small cell lung cancer (NSCLC): a phase II study in elderly or poor performance status patients.
Lung Cancer. 2006; 53(3):355-60 [PubMed] Related Publications
The aim of the study was to assess the activity and tolerability of the combination of gemcitabine (GEM) and vindesine (VDS) in elderly or poor performance patients with advanced non-small cell lung cancer. Forty four patients (36 males and 8 females with a median age of 70 years and a median Karnofsky performance score of 60) were recruited between January 1998 and June 2001; 9 (20.5%) were stage IIIB patients and 35 (79.5%) were stage IV patients; 20 (45.5%) had squamous carcinoma and 24 (54.5%) non-squamous carcinoma. The patients received GEM 1000 mg/m(2) and VDS 3mg/m(2) (max 5mg) on days 1 and 8 every 3 weeks, and were all evaluable for response and toxicity: 17 (38.6%) were partial responders, 17 (38.6%) experienced stable disease, and 10 (22.3%) progressive disease. Grade 3-4 anemia, neutropenia and thrombocytopenia were observed in, respectively, 6.8, 9.1 and 2.3% of the patients, and grade 2-3 fatigue, paresthesias and skin toxicity in, respectively, 11.4, 20.4 and 2.3%. After a median follow-up of 54 months, 43/44 patients died; median survival was 12 months, and a clinical benefit was observed in 54.5% of cases. GEM plus VDS is an active and well-tolerated schedule.
Dooms CA, Lievens YN, Vansteenkiste JF
Cost-utility analysis of chemotherapy in symptomatic advanced nonsmall cell lung cancer.
Eur Respir J. 2006; 27(5):895-901 [PubMed] Related Publications
Cost-utility analysis of chemotherapy in symptomatic advanced nonsmall cell lung cancer.
Eur Respir J. 2006; 27(5):895-901 [PubMed] Related Publications
When using chemotherapy in patients with a short life expectancy, outcomes such as symptom improvement or clinical benefit receive increasing attention. Outcomes of subjective benefit to the patient can be rated as a utility in order to perform health economic analyses and comparisons with other treatment conditions. A cost-utility analysis has been performed alongside a prospective randomised clinical trial comparing single agent gemcitabine to cisplatin-based chemotherapy in symptomatic advanced nonsmall cell lung cancer patients. Global quality of life as well as resource utilisation data were collected during first-line chemotherapy for both treatment arms. Incremental costs, utilities and cost-utility ratio were calculated. Per patient, an incremental cost of 1,522 was obtained for gemcitabine compared to cisplatin-vindesine, mainly as a consequence of the direct cost of the cytotoxic drugs. When combined with utilities, this resulted in an incremental cost-utility ratio for gemcitabine of 13,836 per quality-adjusted life year gained. In conclusion, although the least expensive strategy is cisplatin-vindesine, the greater clinical benefit of gemcitabine, resulting in an acceptable incremental cost-utility ratio as compared with other healthcare interventions, balances its higher cost. The gains in subjective outcome achieved with palliative chemotherapy are critical from both a clinical and a health economic point of view.
Bonn O, Schmidt-Wolf G, Risse F, et al.
Vindesine and etoposide: A practical and well-tolerated therapy for elderly patients or patients in reduced clinical condition with extensive-stage small-cell lung cancer (SCLC).
Med Sci Monit. 2005; 11(2):PI19-21 [PubMed] Related Publications
Vindesine and etoposide: A practical and well-tolerated therapy for elderly patients or patients in reduced clinical condition with extensive-stage small-cell lung cancer (SCLC).
Med Sci Monit. 2005; 11(2):PI19-21 [PubMed] Related Publications
BACKGROUND: Small-cell lung cancer is a disease affecting mostly elderly persons. Therefore, many patients show marked comorbidity and intensive chemotherapy is not possible in such patients. As the disease is most often in the 'extensive' stage at diagnosis, the therapy option is only palliative.
MATERIALS/METHODS: [corrected] We used a combination therapy with vindesine and etoposide in treating 41 patients (median age: 63 years) over 155 cycles of chemotherapy.
RESULTS: This treatment resulted in a response rate of 43.9% and a median survival time of 9.3 months and thus equals established schemes in its effectiveness. However, its rate of adverse effects (hematoxicity, gastrointestinal toxicity, neuropathy) is smaller than those of the established therapy schemes ACO, EPICO, or carboplatine/etoposide.
CONCLUSIONS: Therapy of advanced SCLC with vindesine and etoposide can be applied in an ambulant setting and offers an improved quality of life with equivalent therapeutic effectivity. Therefore it is especially suitable for patients of older age and fragile condition due to comorbidity.
MATERIALS/METHODS: [corrected] We used a combination therapy with vindesine and etoposide in treating 41 patients (median age: 63 years) over 155 cycles of chemotherapy.
RESULTS: This treatment resulted in a response rate of 43.9% and a median survival time of 9.3 months and thus equals established schemes in its effectiveness. However, its rate of adverse effects (hematoxicity, gastrointestinal toxicity, neuropathy) is smaller than those of the established therapy schemes ACO, EPICO, or carboplatine/etoposide.
CONCLUSIONS: Therapy of advanced SCLC with vindesine and etoposide can be applied in an ambulant setting and offers an improved quality of life with equivalent therapeutic effectivity. Therefore it is especially suitable for patients of older age and fragile condition due to comorbidity.
van den Bent MJ
Can chemotherapy replace radiotherapy in low-grade gliomas? Time for randomized studies.
Semin Oncol. 2003; 30(6 Suppl 19):39-44 [PubMed] Related Publications
Can chemotherapy replace radiotherapy in low-grade gliomas? Time for randomized studies.
Semin Oncol. 2003; 30(6 Suppl 19):39-44 [PubMed] Related Publications
For the last three decades surgery and radiotherapy have been the mainstay of treatment for patients with low-grade gliomas, despite a lack of support from randomized controlled trials. Recent developments in our knowledge of low-grade tumor chemosensitivity and the approval of temozolomide for treatment of gliomas have led to increased interest in chemotherapy for treating low-grade gliomas. Despite challenges, including response assessment and appropriate patient selection, several phase II studies investigating chemotherapeutic treatment of low-grade gliomas have yielded promising results. Although most of these phase II studies are of limited sample size, they have shown that chemotherapy might induce clinically relevant responses and disease stabilization in patients with low-grade gliomas. As expected, low-grade oligodendroglioma is sensitive to chemotherapy, but responses were also seen in astrocytic tumors. Randomized, controlled studies should be conducted to determine the clinical significance of responses observed in phase II studies and to assess time to progression. Two randomized, controlled studies are currently investigating chemotherapy in the treatment of low-grade gliomas. Although it will take years before the data are available, these studies will help define the role of chemotherapy in the treatment of low-grade gliomas. Perhaps then we can answer the question, can chemotherapy replace radiotherapy in low-grade gliomas?
André M, Mounier N, Leleu X, et al.
Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients.
Blood. 2004; 103(4):1222-8 [PubMed] Related Publications
Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: a GELA cohort study on 2837 patients.
Blood. 2004; 103(4):1222-8 [PubMed] Related Publications
The survival of patients with aggressive non-Hodgkin lymphoma (NHL) is increasing, but the incidence of secondary cancer and late toxicity is poorly defined for those treated with cyclophosphamide-hydroxydaunomycin/doxorubicin-Oncovin-prednisone (CHOP)-like chemotherapy. From February 1984 to January 1998, 2837 patients with aggressive NHL received the control-arm chemotherapy adriamycin-cyclophosphamide-vindesine-bleomycin-prednisone (ACVBP) in 3 consecutive Groupe d'Etude des Lymphomes de l'Adulte (GELA) studies. With a median follow-up time of 74 months, the 5-year overall and event-free survival rates were 60% and 52%. Two hundred two occurrences of nonneoplastic late toxicity were reported, resulting in a 5.35% cumulative probability of incidence at 7 years. Eighty-one second tumors developed, for which the 7-year cumulative incidence rate was 2.75%; 64 were solid tumors, and 17 were hematologic malignancies. In multivariate analysis, age was the only risk factor for the second development of cancer. Epidemiologic analysis allowed a comparison of this NHL group with the general population. Considering all tumors, no excess of second cancer was observed. In the male population, however, there was an excess of lung cancer (standardized incidence ratio [SIR], 2.45; P <.001) and myelodysplastic syndrome/acute myelocytic leukemia (MDS/AML) (SIR, 5.65; P =.006), and in the female population there was an excess of MDS/AML (SIR, 19.9; P <.001). With a long follow-up, the ACVBP regimen was highly effective for the treatment of aggressive NHL. Increases occurred in secondary MDS/AML and in lung cancer among men.
Merighi S, Mirandola P, Varani K, et al.
Pyrazolotriazolopyrimidine derivatives sensitize melanoma cells to the chemotherapic drugs: taxol and vindesine.
Biochem Pharmacol. 2003; 66(5):739-48 [PubMed] Related Publications
Pyrazolotriazolopyrimidine derivatives sensitize melanoma cells to the chemotherapic drugs: taxol and vindesine.
Biochem Pharmacol. 2003; 66(5):739-48 [PubMed] Related Publications
In this study, we have evaluated the "in vitro" modulatory activity of a series of pyrazolotriazolopyrimidine derivatives (PTP-d) in sensitizing malignant melanoma cells to the chemotherapic drugs: taxol and vindesine. To that end, we have described the impact of chemotherapeutic agents on the cell cycle and on the induction of apoptosis when used alone or in combination with PTP-d. We have demonstrated that four PTP-d reduced chemotherapic drugs EC(50) doses of the G(2)/M accumulation with an average of 1.7-fold for taxol and 9.5-fold for vindesine when challenged on A375 human melanoma cell line. This sensitization activity was also confirmed by analyzing the apoptosis degree induced by the chemotherapic drugs. Interestingly, PTP-d had no effects on the response to cytotoxic agents by skin-derived human keratinocyte cells, NCTC 2544. Therefore, we have investigated the signaling pathway sustaining the sensitizing effect of PTP-d, providing functional evidence that active compounds are able to inhibit multidrug resistance-associated ATP-binding cassette drug transporter. These results suggested that PTP-d hold great promise for the treatment of multidrug resistance in cancers, leading to potential new therapies for melanoma.
Emmert S, Zutt M, Haenssle H, et al.
Inefficacy of vindesine monotherapy in advanced stage IV malignant melanoma patients previously treated with other chemotherapeutic agents.
Melanoma Res. 2003; 13(3):299-302 [PubMed] Related Publications
Inefficacy of vindesine monotherapy in advanced stage IV malignant melanoma patients previously treated with other chemotherapeutic agents.
Melanoma Res. 2003; 13(3):299-302 [PubMed] Related Publications
The anti-melanoma activity of vindesine as a single or polychemotherapeutic agent has been reported previously in adjuvant and first-line melanoma treatment. In this study, we investigated the usefulness of vindesine monotherapy as salvage therapy in stage IV melanoma patients after failure of other chemotherapies. Thirteen patients with progressive disease were treated with 3 mg/m2 vindesine every 2 weeks (median age, 61 years). Previous systemic treatment consisted of polychemotherapy or combined chemo-immunotherapy. All 13 patients suffered from visceral metastases (three lung, one liver, one adrenal gland and eight multiple visceral metastases). A median of three vindesine treatments was administered. Despite the various pre-treatments, the toxicity of vindesine was mild. In all 13 patients, vindesine treatment was stopped due to disease progression. The median survival after primary tumour diagnosis was 42 months (8-151 months), the survival after entering stage IV was 11 months (3-35 months), and the survival after starting vindesine therapy was 4 months (1-22 months). We conclude that vindesine monotherapy is ineffective in stage IV melanoma patients previously treated with other chemotherapeutic agents.
Gebbia V, Galetta D, Riccardi F, et al.
Vinorelbine plus cisplatin versus cisplatin plus vindesine and mitomycin C in stage IIIB-IV non-small cell lung carcinoma: a prospective randomized study.
Lung Cancer. 2002; 37(2):179-87 [PubMed] Related Publications
Vinorelbine plus cisplatin versus cisplatin plus vindesine and mitomycin C in stage IIIB-IV non-small cell lung carcinoma: a prospective randomized study.
Lung Cancer. 2002; 37(2):179-87 [PubMed] Related Publications
PURPOSE: To compare a regimen of vinorelbine and cisplatin (VC) to the combination of mitomycin, vindesine, and cisplatin (MVP) in patients with stage IIIB or stage IV non-small cell lung cancer (NSCLC). The main endpoits were analysis of objective response rates, toxicity, time to progression, and overall survival.
PATIENTS AND METHODS: 247 eligible patients were randomized to receive (a) vinorelbine 25 mg/m(2) intravenous bolus on days 1 and 8 plus cisplatin 100 mg/m(2) on day 1 every 4 weeks, or (b) mitomycin c 8 mg/m(2) i.v. on day 1, vindesine 3 mg/m(2) i.v. on days 1, 8, 15 and 22, plus cisplatin 100 mg/m(2) on day 1 every 4 weeks. In subsequent cycles vindesine was given every other week. For both treatments a maximum of six cycles was planned. Patients with performance status 0-2 according to the ECOG scale were enrolled. Response and toxicity were evaluated according to the WHO criteria. Analysis of clinical efficacy was performed according to an intent-to-treat analysis.
RESULTS: No statistically significant differences in clinical efficacy were observed between the two chemotherapy regimens. The overall objective response rates were 39% (95% CL, 31-49%) in the VC arm and 42% (95% CL, 33-51%) in the MVP arm (P=0.13). Median time to progression was 4.2 and 4.5 months for the MVP arm and the VC arm, respectively. Median overall survival was 7 months in the VC arm and 8 months in the MVP one (log-rank test, P=0.898). These differences were not statistically significant. However, leukopenia and thrombocytopenia were significantly higher in the MVP arm than in the VC (P=0.0001; P=0.0002). Grade 3 alopecia was more frequent in the MVP arm than in the VC one (P<0.001), which was associated with higher rate of phlebitis (P=0.037).
CONCLUSION: Data achieved in this study suggest that the vinorelbine-cisplatin doublet is similar to the three-drug MVP regimen in term of overall response rate, time to progressive disease, and overall survival. However, hematological toxicity and alopecia are more frequent and severe in the MVP regimen which therefore appears to be less tolerable than the VC regimen. The combination of vinorelbine and cisplatin may be considered as a reference treatment for future studies on the treatment of advanced NSCLC.
PATIENTS AND METHODS: 247 eligible patients were randomized to receive (a) vinorelbine 25 mg/m(2) intravenous bolus on days 1 and 8 plus cisplatin 100 mg/m(2) on day 1 every 4 weeks, or (b) mitomycin c 8 mg/m(2) i.v. on day 1, vindesine 3 mg/m(2) i.v. on days 1, 8, 15 and 22, plus cisplatin 100 mg/m(2) on day 1 every 4 weeks. In subsequent cycles vindesine was given every other week. For both treatments a maximum of six cycles was planned. Patients with performance status 0-2 according to the ECOG scale were enrolled. Response and toxicity were evaluated according to the WHO criteria. Analysis of clinical efficacy was performed according to an intent-to-treat analysis.
RESULTS: No statistically significant differences in clinical efficacy were observed between the two chemotherapy regimens. The overall objective response rates were 39% (95% CL, 31-49%) in the VC arm and 42% (95% CL, 33-51%) in the MVP arm (P=0.13). Median time to progression was 4.2 and 4.5 months for the MVP arm and the VC arm, respectively. Median overall survival was 7 months in the VC arm and 8 months in the MVP one (log-rank test, P=0.898). These differences were not statistically significant. However, leukopenia and thrombocytopenia were significantly higher in the MVP arm than in the VC (P=0.0001; P=0.0002). Grade 3 alopecia was more frequent in the MVP arm than in the VC one (P<0.001), which was associated with higher rate of phlebitis (P=0.037).
CONCLUSION: Data achieved in this study suggest that the vinorelbine-cisplatin doublet is similar to the three-drug MVP regimen in term of overall response rate, time to progressive disease, and overall survival. However, hematological toxicity and alopecia are more frequent and severe in the MVP regimen which therefore appears to be less tolerable than the VC regimen. The combination of vinorelbine and cisplatin may be considered as a reference treatment for future studies on the treatment of advanced NSCLC.
Atagi S, Kawahara M, Hosoe S, et al.
A phase II study of continuous concurrent thoracic radiotherapy in combination with mitomycin, vindesine and cisplatin in unresectable stage III non-small cell lung cancer.
Lung Cancer. 2002; 36(1):105-11 [PubMed] Related Publications
A phase II study of continuous concurrent thoracic radiotherapy in combination with mitomycin, vindesine and cisplatin in unresectable stage III non-small cell lung cancer.
Lung Cancer. 2002; 36(1):105-11 [PubMed] Related Publications
The split-course concurrent thoracic radiation therapy (TRT) and full-dose chemotherapy for unresectable stage III non-small cell lung cancer (NSCLC) has produced promising results by comparison with the sequential approach. Instead of split-course radiation, we conducted a phase II study to investigate the feasibility of continuous concurrent TRT and chemotherapy. Twenty-two patients with unresectable NSCLC were enrolled onto a phase II study of continuous concurrent radiotherapy and chemotherapy. Treatment consisted of two courses of cisplatin (80 mg/m(2) on days 1 and 29), vindesine (3 mg/m(2) on days 1, 8, 29 and 36), and mitomycin (8 mg/m(2) on days 1 and 29). TRT began on day 2 at a dose of 60 Gy (2 Gy per fraction and 5 fractions per week for a total of 30 fractions). Of 22 patients assessable for response, none achieved a CR and 17 (77.3%) achieved a PR with an overall response rate of 77.3% (95% confidence interval, 54.6-92.2%). Grade 3 or 4 leukopenia was observed in 5/13 (81.8%) patients. Six patients (27.3%) experienced > or = grade 3 thrombocytopenia. Non-hematological toxicity was relatively mild. The overall median survival time was 19.0 months and the 1- and 2-year survival rates were 84.8 and 34.5%, respectively. It was possible to administer two courses of chemotherapy in 18 patients (81.8%) as planned. Nineteen (86.4%) of the 22 patients received the planned 60 Gy radiation. It seems to be difficult to administer the planned treatment without any interruption for the majority of patients. However, in the selected patients who completed the 60 Gy TRT, nearly half of the patients completed TRT without interruption. This combination regimen is considered to be feasible on condition that the stopping rule of the treatment is followed. We recommend administering radiotherapy continuously as far as possible.
Kunikane H, Watanabe K, Fukuoka M, et al.
Double-blind randomized control trial of the effect of recombinant human erythropoietin on chemotherapy-induced anemia in patients with non-small cell lung cancer.
Int J Clin Oncol. 2001; 6(6):296-301 [PubMed] Related Publications
Double-blind randomized control trial of the effect of recombinant human erythropoietin on chemotherapy-induced anemia in patients with non-small cell lung cancer.
Int J Clin Oncol. 2001; 6(6):296-301 [PubMed] Related Publications
BACKGROUND: We studied the clinical effect of recombinant human erythropoietin (r-huEPO) on anemia induced by two courses of cisplatin-based chemotherapy in patients with non-small cell lung cancer (NSCLC).
METHODS: Seventy-two patients with NSCLC were randomized into three groups, receiving 100, or 200 IU/kg of r-huEPO, or placebo. The r-huEPO and placebo were administered subcutaneously three times a week for 6 weeks, starting 2 weeks after the initiation of chemotherapy.
RESULTS: In the 53 evaluable patients, hemoglobin (Hb) levels at the nadir after the second cycle of chemotherapy were significantly elevated compared with the nadir after the first cycle in both r-huEPO treated groups, while this level was decreased in the placebo group. Hb levels at the end of the second course of chemotherapy (week 8) in both r-huEPO groups were higher than that in the placebo groups. No adverse drug reaction attributable to r-huEPO was observed. Serum erythropoietin levels after the administration of r-huEPO were higher than those after placebo administration.
CONCLUSIONS: r-huEPO had an effect in preventing anemia in patients with NSCLC who had cisplatin-based chemotherapy.
METHODS: Seventy-two patients with NSCLC were randomized into three groups, receiving 100, or 200 IU/kg of r-huEPO, or placebo. The r-huEPO and placebo were administered subcutaneously three times a week for 6 weeks, starting 2 weeks after the initiation of chemotherapy.
RESULTS: In the 53 evaluable patients, hemoglobin (Hb) levels at the nadir after the second cycle of chemotherapy were significantly elevated compared with the nadir after the first cycle in both r-huEPO treated groups, while this level was decreased in the placebo group. Hb levels at the end of the second course of chemotherapy (week 8) in both r-huEPO groups were higher than that in the placebo groups. No adverse drug reaction attributable to r-huEPO was observed. Serum erythropoietin levels after the administration of r-huEPO were higher than those after placebo administration.
CONCLUSIONS: r-huEPO had an effect in preventing anemia in patients with NSCLC who had cisplatin-based chemotherapy.
Sekine I, Nishiwaki Y, Ogino T, et al.
Phase II study of twice-daily high-dose thoracic radiotherapy alternating with cisplatin and vindesine for unresectable stage III non-small-cell lung cancer: Japan Clinical Oncology Group Study 9306.
J Clin Oncol. 2002; 20(3):797-803 [PubMed] Related Publications
Phase II study of twice-daily high-dose thoracic radiotherapy alternating with cisplatin and vindesine for unresectable stage III non-small-cell lung cancer: Japan Clinical Oncology Group Study 9306.
J Clin Oncol. 2002; 20(3):797-803 [PubMed] Related Publications
PURPOSE: To evaluate the efficacy and toxicity of high-dose thoracic radiotherapy (TRT) alternating with chemotherapy (CH) for unresectable stage III non--small-cell lung cancer (NSCLC).
PATIENTS AND METHODS: Forty-one patients received TRT with 1.5 Gy twice daily, 5 days a week, on weeks 1, 2, 5, 6, and 9, up to a total dose of 66 to 72 Gy, alternating with cisplatin 80 mg/m(2) on day 1 and vindesine 3 mg/m(2) on days 1 and 8, repeated every 4 weeks, for two or three courses beginning on week 3.
RESULTS: The median (range) total dose of TRT and number of CH courses were 72 Gy (16.5 to 72 Gy) and three (zero to three), respectively. Delay in TRT > or = 5 days was observed in 24 (75%) of 32 patients who completed the projected treatment, due to leukopenia in 12, esophagitis in seven, infection in two, and other causes in three patients. Partial responses were obtained in 36 patients (88%). The median survival time and 3- and 5-year survival rates were 18.4 months, 24%, and 10%, respectively. Grade 3 or 4 leukopenia and esophagitis developed in 32 and seven patients, respectively. Grade 3 or 4 late esophageal toxicity developed in two patients.
CONCLUSION: Alternating high-dose TRT and CH for stage III NSCLC produced a high response rate with median and long-term survival comparable to prior trials utilizing standard approaches in this population. Acute and late esophageal toxicity was observed and interruption of TRT was required in most of the patients.
PATIENTS AND METHODS: Forty-one patients received TRT with 1.5 Gy twice daily, 5 days a week, on weeks 1, 2, 5, 6, and 9, up to a total dose of 66 to 72 Gy, alternating with cisplatin 80 mg/m(2) on day 1 and vindesine 3 mg/m(2) on days 1 and 8, repeated every 4 weeks, for two or three courses beginning on week 3.
RESULTS: The median (range) total dose of TRT and number of CH courses were 72 Gy (16.5 to 72 Gy) and three (zero to three), respectively. Delay in TRT > or = 5 days was observed in 24 (75%) of 32 patients who completed the projected treatment, due to leukopenia in 12, esophagitis in seven, infection in two, and other causes in three patients. Partial responses were obtained in 36 patients (88%). The median survival time and 3- and 5-year survival rates were 18.4 months, 24%, and 10%, respectively. Grade 3 or 4 leukopenia and esophagitis developed in 32 and seven patients, respectively. Grade 3 or 4 late esophageal toxicity developed in two patients.
CONCLUSION: Alternating high-dose TRT and CH for stage III NSCLC produced a high response rate with median and long-term survival comparable to prior trials utilizing standard approaches in this population. Acute and late esophageal toxicity was observed and interruption of TRT was required in most of the patients.
Obara K, Ghazizadeh M, Shimizu H, et al.
Comparative genomic hybridization study of genetic changes associated with vindesine resistance in esophageal carcinoma.
Int J Oncol. 2002; 20(2):255-60 [PubMed] Related Publications
Comparative genomic hybridization study of genetic changes associated with vindesine resistance in esophageal carcinoma.
Int J Oncol. 2002; 20(2):255-60 [PubMed] Related Publications
The acquisition of drug-resistance is a major problem for cancer patients undergoing chemotherapy. To clarify genetic alterations in cancer cells that develop drug-resistance, comparative genomic hybridization (CGH) was applied to esophageal squamous cell carcinoma cell lines (SH-1V1, SH-1V2, SH-1V4 and SH-1V8) and chemoresistance-related genes in altered chromosomal regions were evaluated. These cell lines were derived from the parental SH-1 cell line, after multiple steps of selection by an increasing exposure to vindesine. SH-1V8 cells were strongly resistant to vindesine. DNA copy number at 16p which includes the MRP (multidrug resistance related protein) gene was markedly increased in all cell lines examined. Increased DNA copy numbers were found at the regions of 5q31-32, 10q11.1-23, and 14q32-qter in SH-1V8 cells that acquired resistance to other drugs as well. Both SH-1V4 and SH-1V8 showed increased DNA copy numbers at 7q11.1-22, 16q12.1-qter, 19p13.2-13.3, 19q11-13.2 and 20q13.1-qter. The chromosomal region of 7q11.1-22 including MDR-1 (multidrug resistance-1) gene was highly amplified in SH-1V4 and SH-1V8. Amplification of the MRP region suggests the prerequisite of developing resistance to vindesine, and further amplification of MDR-1 may play a critical role in acquiring drug-resistance. Several unknown genes related to the induction of chemoresistance might be concealed in other altered chromosomal regions.
Kawakami Y, Hama S, Hiura M, et al.
Adenovirus-mediated p16 gene transfer changes the sensitivity to taxanes and Vinca alkaloids of human ovarian cancer cells.
Anticancer Res. 2001 Jul-Aug; 21(4A):2537-45 [PubMed] Related Publications
Adenovirus-mediated p16 gene transfer changes the sensitivity to taxanes and Vinca alkaloids of human ovarian cancer cells.
Anticancer Res. 2001 Jul-Aug; 21(4A):2537-45 [PubMed] Related Publications
BACKGROUND: Deletions and point mutations of the p16 gene are detectable in more than 50% of ovarian cancer cells. In this study, we examined the effect of p16 gene transduction on the growth of ovarian cancer cells and on the effect of anti-cancer agents.
MATERIALS AND METHODS: p16-null human ovarian cancer cell lines, SKOV-3 and OVCAR-5, were used in this study. We transduced the full-length human p16 gene using recombinant adenovirus (AxCA-hp16).
RESULTS: The spontaneous growth of these cells was significantly inhibited by hp16 transduction. MTT assay revealed that AxCA-hp16 infection induced chemoresistance in both cell lines. Flow cytometric analysis revealed that only hp16 -transduced SKOV-3, were arrested at the G1-phase for 3 days whereas those infected with AxCA-mock and OVCAR-5 infected with both recombinant viruses did not. Western blot analysis showed increased microtubule-associated proteins 4 (MAP4) in both cell lines.
CONCLUSION: These results suggest that in SKOV-3 cells, G1-arrest induced by p16-transduction prevents paclitaxel- and vindesine-induced cell death, and in OVCAR-5 cells, the other unknown mechanisms play a role of chemoresistance.
MATERIALS AND METHODS: p16-null human ovarian cancer cell lines, SKOV-3 and OVCAR-5, were used in this study. We transduced the full-length human p16 gene using recombinant adenovirus (AxCA-hp16).
RESULTS: The spontaneous growth of these cells was significantly inhibited by hp16 transduction. MTT assay revealed that AxCA-hp16 infection induced chemoresistance in both cell lines. Flow cytometric analysis revealed that only hp16 -transduced SKOV-3, were arrested at the G1-phase for 3 days whereas those infected with AxCA-mock and OVCAR-5 infected with both recombinant viruses did not. Western blot analysis showed increased microtubule-associated proteins 4 (MAP4) in both cell lines.
CONCLUSION: These results suggest that in SKOV-3 cells, G1-arrest induced by p16-transduction prevents paclitaxel- and vindesine-induced cell death, and in OVCAR-5 cells, the other unknown mechanisms play a role of chemoresistance.
Colombi M, Guffanti A, Alietti A, et al.
OPP-EBV-CAD regimen as salvage treatment in advanced refractory or resistant multiple myeloma.
Leuk Lymphoma. 2000; 40(1-2):87-94 [PubMed] Related Publications
OPP-EBV-CAD regimen as salvage treatment in advanced refractory or resistant multiple myeloma.
Leuk Lymphoma. 2000; 40(1-2):87-94 [PubMed] Related Publications
With the aim of developing an effective therapy for heavily pretreated refractory MM outpatients, we evaluated the OPPEBVCAD regimen, a Hodgkin's disease-derived protocol that includes many drugs effective in MM administered in a sequential schedule. Twenty-two pts aged 42-72 years, with symptomatic highly-pretreated refractory (18 cases), or primary resistant MM (four cases. including two pts with plasma cell leukemia-PCL) received this therapy every 28 days (2-4 cycles, followed by a maintenance program). Therapeutic response (Chronic Leukemia-Myeloma Task Force criteria) and performance status (PS) and pain (W.H.O.) were evaluated. All of the pts were evaluable for response. There were 9 (40%) objective responses (OR: stabilization of blood counts and bone lesions, serum calcium normalization, 50% or more reduction in the concentration of serum monoclonal component (MC), 90% reduction in Bence-Jones proteinuria), 8 (36%) partial responses (PR: 25-50% reduction in serum MC), 1 no response or stable disease (NR), and 4 (18%) cases of progressive disease (PD). OR plus PR were 77%. Of the 4 primary resistant tumors (2 PCL and 2 MM), 2 achieved PR, 1 OR (a PCL case) and 1 progressed. Median survival was 15 months for responding pts (OR plus PR) and 4.5 months for non-responders (NR plus PD). PS and pain improved in 15 pts and did not change in 9. The most frequent side effects were cytopenias, with one drug related infective death. The OPPEBVCAD regimen proved to be an effective therapy for refractory relapsing or primary resistant MM: in responders (two-thirds of the pts), survival was prolonged by about 10 months. Its efficacy in anthracycline-treated pts, as well as the feasibility of using it on an outpatient basis without any continuous drug infusions, make this regimen a promising third line salvage therapy.
Nishio K, Nakamura T, Koh Y, et al.
Oncoprotein 18 overexpression increases the sensitivity to vindesine in the human lung carcinoma cells.
Cancer. 2001; 91(8):1494-9 [PubMed] Related Publications
Oncoprotein 18 overexpression increases the sensitivity to vindesine in the human lung carcinoma cells.
Cancer. 2001; 91(8):1494-9 [PubMed] Related Publications
BACKGROUND: Oncoprotein 18 (op18) was first isolated as a molecule overexpressed in several malignant cells, suggesting a function of op18 in malignant processes, such as differentiation in hematologic malignancies, op18 also was found to enhance microtubule deassembly in the cells. Antimitotic agents that bind to tubulin have been used for chemotherapy to treat solid tumors, such as lung carcinoma. Vinca alkaloids, such as vindesine and vincristine, have commonly been used for chemotherapy of nonsmall cell lung carcinoma. The authors examined the role of op18 in the sensitivity of human lung carcinoma cells to antimitotic agents.
METHODS: Expression of op18 mRNA was detected in all 17 lung carcinoma cell lines tested by Northern blotting. Oncoprotein 18 cDNA was transfected to SBC-3 human lung carcinoma cells, and the stable transfectants, SBC-3/op1-3, were isolated. The sensitivity of these transfectants against antimitotic agents were examined by the MTT assay in vitro. Cell cycle distribution of the transfectants on DNA histogram was analyzed by flow cytometry.
RESULTS: Oncoprotein 18-transfected cells showed higher sensitivity to vindesine and vincristine, but not to taxanes. Vindesine-exposure increased the G2/M population of the cell cycle in the Mock transfectants, but not in SBC-3/op1, suggesting that the cell cycle dynamics were altered by op18 expression in SBC-3/op1.
CONCLUSION: Oncoprotein 18 expression is associated with lung carcinoma cell sensitivity to vindesine and may be able to serve as a surrogate marker for the chemosensitivity to Vinca alkaloids in human lung carcinomas.
METHODS: Expression of op18 mRNA was detected in all 17 lung carcinoma cell lines tested by Northern blotting. Oncoprotein 18 cDNA was transfected to SBC-3 human lung carcinoma cells, and the stable transfectants, SBC-3/op1-3, were isolated. The sensitivity of these transfectants against antimitotic agents were examined by the MTT assay in vitro. Cell cycle distribution of the transfectants on DNA histogram was analyzed by flow cytometry.
RESULTS: Oncoprotein 18-transfected cells showed higher sensitivity to vindesine and vincristine, but not to taxanes. Vindesine-exposure increased the G2/M population of the cell cycle in the Mock transfectants, but not in SBC-3/op1, suggesting that the cell cycle dynamics were altered by op18 expression in SBC-3/op1.
CONCLUSION: Oncoprotein 18 expression is associated with lung carcinoma cell sensitivity to vindesine and may be able to serve as a surrogate marker for the chemosensitivity to Vinca alkaloids in human lung carcinomas.
Cazals-Hatem D, André M, Mounier N, et al.
Pathologic and clinical features of 77 Hodgkin's lymphoma patients treated in a lymphoma protocol (LNH87): a GELA study.
Am J Surg Pathol. 2001; 25(3):297-306 [PubMed] Related Publications
Pathologic and clinical features of 77 Hodgkin's lymphoma patients treated in a lymphoma protocol (LNH87): a GELA study.
Am J Surg Pathol. 2001; 25(3):297-306 [PubMed] Related Publications
Between 1987 and 1993, 77 of 2855 lymphomas included in the LNH87 protocol of the GELA as non-Hodgkin lymphoma (NHL) and reviewed by a panel of pathologists had a diagnosis changed to Hodgkin lymphoma (HL). Some of these lymphomas had been initially interpreted as anaplastic large-cell lymphoma Hodgkin-like (ALCL-HL subtype). The purpose of this study was to analyze the histologic pitfalls initially encountered, to define more clearly the diagnostic criteria of lymphomas placed in the gray zone around HL, and to follow the survival of these 77 patients affected with HL and initially treated with NHL regimens. The 77 cases of HL were reviewed by three hematopathologists and immunostained with a large panel of antibodies, including CD30, CD15, CD3, CD20, CD45, CD43, LMP-1, EMA, BNH-9, TiA1, and ALK1. Each case was classified according to the Lukes-Rye system and the British National Lymphoma Investigation (BNLI) grading. The initial clinical presentation of patients was analyzed, and the overall and event-free survival rates of the 77 patients were estimated. Among the 77 HLs, 46 were misinterpreted as NHL by primary individual pathologists (12 as ALCL, 8 as ALCL-HL, 12 as peripheral T-cell lymphoma (PTCL), 6 as B-cell lymphoma, and 8 as unclassifiable NHL). The other 31 cases had been first considered by the panel as consistent with ALCL-HL (n = 18) or with PTCL (n = 13) and were changed later in view of an immunophenotype concordant with HL. Fifty-five percent of the patients completed the full NHL treatment. The 5-year event-free and overall survival rates were 54% and 77%, respectively. The current results indicate that lymphomas initially called ALCL-HL should not be regarded as a variant of ALCL, but as HL. The clinical consequences of misdiagnoses seem to be a lower event-free survival rate compared with that of classical HL, probably because of more relapses of initially inappropriately treated HL.
Takigawa N, Segawa Y, Ueoka H, et al.
Combination of nedaplatin and vindesine for treatment of relapsed or refractory non-small-cell lung cancer.
Cancer Chemother Pharmacol. 2000; 46(4):272-8 [PubMed] Related Publications
Combination of nedaplatin and vindesine for treatment of relapsed or refractory non-small-cell lung cancer.
Cancer Chemother Pharmacol. 2000; 46(4):272-8 [PubMed] Related Publications
PURPOSE: A phase II study of nedaplatin and vindesine was conducted to evaluate their efficacy and safety for treatment of relapsed or refractory non-small-cell lung cancer (NSCLC).
METHODS: Between August 1996 and September 1998, 48 patients who had previously received chemotherapy, thoracic radiotherapy, and/or surgery were enrolled in the study. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2 and an age between 20 and 79 years. Treatment consisted of nedaplatin (80 mg/m2, day 1) and vindesine (3 mg/m2, days 1 and 8) every 3 to 4 weeks.
RESULTS: Of 48 patients, 7 (14.6%) exhibited an objective response. Four (50%) of eight chemotherapy-naive patients had a partial response. However, of the 40 patients who had received prior chemotherapy, a partial response was observed in only 3 (7.5%). At a median follow-up time of 85.1 weeks, the median survival time was 43.6 weeks (95% confidence interval 34.4-52.7) for patients who had received chemotherapy, with a survival rate of 40% at 1 year. Grade 3 or 4 neutropenia occurred in 43 of 48 patients (90%), and neutropenic fever was observed in 3 of the 43 patients, one of whom died of sepsis. Pharmacokinetic and pharmacodynamic analyses of platinum were performed in 43 patients during the first cycle of chemotherapy. Percent reduction in absolute neutrophil count was correlated not only with the area under the plasma ultrafilterable platinum concentration versus time curve (r = 0.41, P = 0.007) but also with the duration of ultrafilterable platinum concentration above 1 microg/ml (r = 0.41, P = 0.007). Patients with progressive disease exhibited a shorter duration of ultrafilterable platinum concentration over 1 microg/ml (P = 0.046) than those with other responses.
CONCLUSION: A combination of nedaplatin and vindesine was unsatisfactory as second-line chemotherapy for NSCLC, although the combination was well tolerated. The duration of ultrafilterable platinum concentration above 1 microg/ml was an important pharmacokinetic parameter for predicting both chemotherapy-induced neutropenia and treatment outcome.
METHODS: Between August 1996 and September 1998, 48 patients who had previously received chemotherapy, thoracic radiotherapy, and/or surgery were enrolled in the study. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2 and an age between 20 and 79 years. Treatment consisted of nedaplatin (80 mg/m2, day 1) and vindesine (3 mg/m2, days 1 and 8) every 3 to 4 weeks.
RESULTS: Of 48 patients, 7 (14.6%) exhibited an objective response. Four (50%) of eight chemotherapy-naive patients had a partial response. However, of the 40 patients who had received prior chemotherapy, a partial response was observed in only 3 (7.5%). At a median follow-up time of 85.1 weeks, the median survival time was 43.6 weeks (95% confidence interval 34.4-52.7) for patients who had received chemotherapy, with a survival rate of 40% at 1 year. Grade 3 or 4 neutropenia occurred in 43 of 48 patients (90%), and neutropenic fever was observed in 3 of the 43 patients, one of whom died of sepsis. Pharmacokinetic and pharmacodynamic analyses of platinum were performed in 43 patients during the first cycle of chemotherapy. Percent reduction in absolute neutrophil count was correlated not only with the area under the plasma ultrafilterable platinum concentration versus time curve (r = 0.41, P = 0.007) but also with the duration of ultrafilterable platinum concentration above 1 microg/ml (r = 0.41, P = 0.007). Patients with progressive disease exhibited a shorter duration of ultrafilterable platinum concentration over 1 microg/ml (P = 0.046) than those with other responses.
CONCLUSION: A combination of nedaplatin and vindesine was unsatisfactory as second-line chemotherapy for NSCLC, although the combination was well tolerated. The duration of ultrafilterable platinum concentration above 1 microg/ml was an important pharmacokinetic parameter for predicting both chemotherapy-induced neutropenia and treatment outcome.
Levin VA, Uhm JH, Jaeckle KA, et al.
Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme.
Clin Cancer Res. 2000; 6(10):3878-84 [PubMed] Related Publications
Phase III randomized study of postradiotherapy chemotherapy with alpha-difluoromethylornithine-procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, vincristine (DFMO-PCV) versus PCV for glioblastoma multiforme.
Clin Cancer Res. 2000; 6(10):3878-84 [PubMed] Related Publications
Although the efficacy of the nitrosourea-based combination chemotherapy procarbazine, N-(2-chloroethyl)-N'-cyclohexyl-N-nitrosurea, and vincristine (PCV) has been previously demonstrated in the setting of anaplastic/intermediate-grade gliomas, the benefit for glioblastoma patients remains unproven. In the current study, we sought to determine whether the addition of alpha-difluoromethylornithine (eflornithine), an inhibitor of ornithine decarboxylase, which has shown encouraging results in the setting of recurrent glioma patients, to a nitrosourea-based therapy (PCV) would constitute a more effective adjuvant therapy in the treatment of glioblastoma multiforme patients in the postradiation therapy setting. Following conventional radiation therapy, 272 glioblastoma (GBM) patients were randomized to receive either alpha-difluoromethylornithine-PCV (DFMO-PCV; 134 patients) or PCV alone (138 patients), with survival and time to tumor progression being the primary endpoints. The starting dosage of DFMO was 3.0 g/m2 p.o. q8h for 14 days before and after treatment with N-(2-chloroethyl)-N-cyclohexyl-N-nitrosurea; PCV was administered as previously described1. Clinical and radiological (Gadolinium-enhanced MRI) follow-ups were nominally at the end of each 6 or 8 week cycle (PCV at 6 weeks; DFMO-PCV at 8 weeks). Laboratory evaluations for hematologic and other adverse effects were at 2 week intervals. There was no difference in median survival or median time-to-tumor progression between the two treatment groups, as measured from day of commencement of postradiotherapy chemotherapy [MS (months): DFMO-PCV, 10.5; Overall survival, as measured from time of tumor diagnosis at first surgery, was 13.3 and 14.2 months at the median and 6.2 and 8.7% at 5 years, respectively, for the DFMO-PCV and PCV arms. The treatment effect was unchanged after adjustment for age, performance status (KPS), extent of surgery, and other factors using the multivariate Cox proportional hazard model. Adverse effects associated with DFMO consisted of gastrointestinal (diarrhea nausea/vomiting), cytopenias, and minimal ototoxicity (limited to tinnitus) at the dose range tested. The addition of DFMO to the nitrosourea-based PCV regimen in this phase III study demonstrated no additional benefit in glioblastoma patients, underscoring the resistance of glioblastoma multiforme tumors to alkylating agents. For patients with anaplastic (intermediate grade) gliomas, in which the previously demonstrated benefit of post-radiation chemotherapy is more substantial, the evaluation of DFMO-PCV vs. PCV is still ongoing and hopefully will yield more encouraging results.
Iqbal M, Marshall E, Green JA
Ten-year survival in advanced malignant melanoma following treatment with interferon and vindesine.
Ann Oncol. 2000; 11(4):483-5 [PubMed] Related Publications
Ten-year survival in advanced malignant melanoma following treatment with interferon and vindesine.
Ann Oncol. 2000; 11(4):483-5 [PubMed] Related Publications
A 31-year-old man with malignant melanoma of his right popliteal fossa was treated in 1987 with surgical excision followed by local radiotherapy. Eight months later, he presented with recurrence in the right inguinal lymph nodes, which were resected and followed by radiotherapy to the groin. Ten months later, he developed liver metastases and was treated with vindesine (12 months) and interferon-alpha-2a (30 months) resulting in complete remission which has been maintained for over 10 years. This interesting case report, with brief review of literature, is presented here.
Recchia F, De Filippis S, Pompili PL, et al.
Carboplatin, vindesine, 5-fluorouracil-leucovorin and 13-cis retinoic acid in the treatment of advanced non-small cell lung cancer. A phase II study.
Clin Ter. 1999 Jul-Aug; 150(4):269-74 [PubMed] Related Publications
Carboplatin, vindesine, 5-fluorouracil-leucovorin and 13-cis retinoic acid in the treatment of advanced non-small cell lung cancer. A phase II study.
Clin Ter. 1999 Jul-Aug; 150(4):269-74 [PubMed] Related Publications
PURPOSE: Carboplatin, vindesine and 5-fluorouracil/leucovorin are drugs active in the treatment of non-small cell lung cancer (NSCLC) and they can be administered in an outpatient setting. Retinoids, which are widely used agents in chemoprevention, have been reported to exert (in vitro models) growth inhibitory effects of synergistic type with chemotherapy, and differentiating effects on NSCLC cells.
PATIENTS AND METHODS: 28 patients with advanced NSCLC with measurable disease were entered into the trial. Eligibility criteria included performance status < or = 3 and adequate renal and liver function. Patients with brain metastases were not excluded. Treatment was as follows: Carboplatin (CBCDA) 300 mg/m2 day 1, Vindesine (VDS) 3 mg/m2 days 1 and 5, leucovorin (L) 100 mg/m2, 5-fluorouracil (5-FU) 370 mg/m2 for 5 days and 13-cis retinoic acid (R) 1 mg/kg, administered between chemotherapy courses. After 6 courses of chemotherapy responders were maintained with R, until progression.
RESULTS: 120 courses of chemotherapy have been delivered (median 4 courses per patient, range 1 to 6). All patients were evaluable for response and toxicity. Objective responses: 2 complete responses (CR) (7%), 9 partial responses (PR) (32%), 9 stable disease (SD) (32%), 8 progressive disease (PD) (29%). (Response rate 39%, 95% CI: 22% to 60%). Median time to progression was 7.7 months (range 3.4-22) and median survival was 9.7 months (range 0.5-27) with 40% of patients alive after one year. Toxicity WHO: hematological grade 3-4 in 46% of patients, grade 2 diarrhea in 21% of patients, ileus in 14% of patients, Neurologic grade 2 in 11% of patients.
CONCLUSIONS: The addition of RA to CBDCA, VDS, FU, L, R represents an effective treatment in NSCLC, with manageable toxicity.
PATIENTS AND METHODS: 28 patients with advanced NSCLC with measurable disease were entered into the trial. Eligibility criteria included performance status < or = 3 and adequate renal and liver function. Patients with brain metastases were not excluded. Treatment was as follows: Carboplatin (CBCDA) 300 mg/m2 day 1, Vindesine (VDS) 3 mg/m2 days 1 and 5, leucovorin (L) 100 mg/m2, 5-fluorouracil (5-FU) 370 mg/m2 for 5 days and 13-cis retinoic acid (R) 1 mg/kg, administered between chemotherapy courses. After 6 courses of chemotherapy responders were maintained with R, until progression.
RESULTS: 120 courses of chemotherapy have been delivered (median 4 courses per patient, range 1 to 6). All patients were evaluable for response and toxicity. Objective responses: 2 complete responses (CR) (7%), 9 partial responses (PR) (32%), 9 stable disease (SD) (32%), 8 progressive disease (PD) (29%). (Response rate 39%, 95% CI: 22% to 60%). Median time to progression was 7.7 months (range 3.4-22) and median survival was 9.7 months (range 0.5-27) with 40% of patients alive after one year. Toxicity WHO: hematological grade 3-4 in 46% of patients, grade 2 diarrhea in 21% of patients, ileus in 14% of patients, Neurologic grade 2 in 11% of patients.
CONCLUSIONS: The addition of RA to CBDCA, VDS, FU, L, R represents an effective treatment in NSCLC, with manageable toxicity.
De Marinis F, Rinaldi M, Ardizzoni A, et al.
The role of vindesine and lonidamine in the treatment of elderly patients with advanced non-small cell lung cancer: a phase III randomized FONICAP trial. Italian Lung Cancer Task Force.
Tumori. 1999 May-Jun; 85(3):177-82 [PubMed] Related Publications
The role of vindesine and lonidamine in the treatment of elderly patients with advanced non-small cell lung cancer: a phase III randomized FONICAP trial. Italian Lung Cancer Task Force.
Tumori. 1999 May-Jun; 85(3):177-82 [PubMed] Related Publications
AIMS: To evaluate the efficacy and treatment compliance in elderly patients with advanced non-small cell lung cancer (NSCLC) of two chemotherapeutic agents with mild toxicity, 153 previously untreated patients aged over 70 years were randomized to receive lonidamine (450 mg daily p.o. until progression), vindesine (3 mg/m2/daily i.v. weekly for 4 weeks and then every 2 weeks until progression), the combination of the two drugs at the same dose and schedule, or supportive therapy only in a four-arm factorial randomized trial.
METHODS: 126 patients were included in the final analysis. Their median age was 75 years. Forty percent had stage IV disease and 60% stage III. Most patients were males (85%) and the majority had squamous histology (68%).
RESULTS: Among 104 patients evaluable for response there were only 3 PRs (1/30 in the lonidamine arm and 2/33 in the lonidamine + vindesine arm). Overall, 8.7% and 9.5% of the patients, respectively, progressed or died early, before response evaluation; another 9.4% refused treatment continuation because of poor compliance with the study protocol. Eighty-five patients were fully evaluable for toxicity, which was generally mild. Leukopenia grade 1-3 was found in less than 30% of patients treated with vindesine or vindesine + lonidamine. The most common complaints associated with lonidamine treatment were myalgia (70% of patients), fatigue (55% and 83% of patients treated with lonidamine or lonidamine + vindesine, respectively) and testicular pain in nearly 40% of cases. The overall median survival was 170 days, with no significant impact on survival of either lonidamine or vindesine.
CONCLUSIONS: The low response rate and survival together with the poor treatment compliance, even in the presence of mild toxicity, do not support the usefulness of these "gentle" chemotherapies in elderly NSCLC patients. The standard management of advanced NSCLC in elderly patients remains to be defined. Specifically designed studies to address this issue are warranted.
METHODS: 126 patients were included in the final analysis. Their median age was 75 years. Forty percent had stage IV disease and 60% stage III. Most patients were males (85%) and the majority had squamous histology (68%).
RESULTS: Among 104 patients evaluable for response there were only 3 PRs (1/30 in the lonidamine arm and 2/33 in the lonidamine + vindesine arm). Overall, 8.7% and 9.5% of the patients, respectively, progressed or died early, before response evaluation; another 9.4% refused treatment continuation because of poor compliance with the study protocol. Eighty-five patients were fully evaluable for toxicity, which was generally mild. Leukopenia grade 1-3 was found in less than 30% of patients treated with vindesine or vindesine + lonidamine. The most common complaints associated with lonidamine treatment were myalgia (70% of patients), fatigue (55% and 83% of patients treated with lonidamine or lonidamine + vindesine, respectively) and testicular pain in nearly 40% of cases. The overall median survival was 170 days, with no significant impact on survival of either lonidamine or vindesine.
CONCLUSIONS: The low response rate and survival together with the poor treatment compliance, even in the presence of mild toxicity, do not support the usefulness of these "gentle" chemotherapies in elderly NSCLC patients. The standard management of advanced NSCLC in elderly patients remains to be defined. Specifically designed studies to address this issue are warranted.



 Cancer Prevention and Risk Reduction
Cancer Prevention and Risk Reduction